Ultrastructure of a novel bacterial form located in Staphylococcus aureus in vitro and in vivo catheter-associated biofilms
- PMID: 22821688
- PMCID: PMC3524562
- DOI: 10.1369/0022155412457573
Ultrastructure of a novel bacterial form located in Staphylococcus aureus in vitro and in vivo catheter-associated biofilms
Abstract
Bacterial biofilms are ubiquitous in nature, industry, and medicine, and understanding their development and cellular structure is critical in controlling the unwanted consequences of biofilm growth. Here, we report the ultrastructure of a novel bacterial form observed by scanning electron microscopy in the luminal vegetations of catheters from patients with active Staphylococcus aureus bacteremia. This novel structure had the general appearance of a normal staphylococcal cell but up to 10 to 15 times as large. Transmission electron microscopy indicated that these structures appeared as sacs enclosing multiple normal-sized (~0.6 µm) staphylococcal forms. Using in vitro cultivated biofilms, cytochemical studies using fluorescent reagents revealed that these structures were rich in lipids and appeared within 15 min after S. aureus inoculation onto clinically relevant abiotic surfaces. Because they appeared early in biofilm development, these novel bacterial forms may represent an unappreciated mechanism for biofilm surface adherence, and their prominent lipid expression levels could explain the perplexing increased antimicrobial resistance of biofilm-associated bacteria.
Conflict of interest statement
Figures

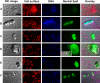

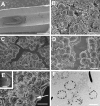
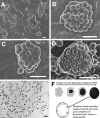
Similar articles
-
Catheter lock solutions influence staphylococcal biofilm formation on abiotic surfaces.Nephrol Dial Transplant. 2006 Aug;21(8):2247-55. doi: 10.1093/ndt/gfl170. Epub 2006 Apr 20. Nephrol Dial Transplant. 2006. PMID: 16627606
-
Antibiosis interaction of Staphylococccus aureus on Aspergillus fumigatus assessed in vitro by mixed biofilm formation.BMC Microbiol. 2015 Feb 15;15:33. doi: 10.1186/s12866-015-0363-2. BMC Microbiol. 2015. PMID: 25880740 Free PMC article.
-
Bacterial contamination of surgical suture resembles a biofilm.Surg Infect (Larchmt). 2010 Oct;11(5):433-9. doi: 10.1089/sur.2010.006. Surg Infect (Larchmt). 2010. PMID: 20673144 Free PMC article.
-
Effect of alkaline pH on staphylococcal biofilm formation.APMIS. 2012 Sep;120(9):733-42. doi: 10.1111/j.1600-0463.2012.02900.x. Epub 2012 Apr 11. APMIS. 2012. PMID: 22882263
-
Staphylococcal biofilms: quest for the magic bullet.Adv Appl Microbiol. 2012;81:63-87. doi: 10.1016/B978-0-12-394382-8.00002-2. Adv Appl Microbiol. 2012. PMID: 22958527 Review.
Cited by
-
Use of epifluorescence widefield deconvolution microscopy for imaging and three-dimensional rendering of Pseudomonas aeruginosa biofilms and extracellular matrix materials.Methods Microbiol. 2023;53:309-324. doi: 10.1016/bs.mim.2023.05.005. Epub 2023 Jun 26. Methods Microbiol. 2023. PMID: 38974073 Free PMC article. No abstract available.
-
Sonochemical fabrication of gradient antibacterial materials based on Cu-Zn alloy.Ultrason Sonochem. 2023 Jan;92:106247. doi: 10.1016/j.ultsonch.2022.106247. Epub 2022 Dec 5. Ultrason Sonochem. 2023. PMID: 36508894 Free PMC article.
-
Biofilm Cohesive Strength as a Basis for Biofilm Recalcitrance: Are Bacterial Biofilms Overdesigned?Microbiol Insights. 2016 Jan 18;8(Suppl 2):29-32. doi: 10.4137/MBI.S31444. eCollection 2015. Microbiol Insights. 2016. PMID: 26819559 Free PMC article.
-
The Natural Surfactant Glycerol Monolaurate Significantly Reduces Development of Staphylococcus aureus and Enterococcus faecalis Biofilms.Surg Infect (Larchmt). 2015 Oct;16(5):538-42. doi: 10.1089/sur.2014.162. Epub 2015 Jun 25. Surg Infect (Larchmt). 2015. PMID: 26110557 Free PMC article.
-
Antibacterial synergy of glycerol monolaurate and aminoglycosides in Staphylococcus aureus biofilms.Antimicrob Agents Chemother. 2014 Nov;58(11):6970-3. doi: 10.1128/AAC.03672-14. Epub 2014 Sep 2. Antimicrob Agents Chemother. 2014. PMID: 25182634 Free PMC article.
References
-
- Cogan NG, Gunn JS, Wozniak DJ. 2011. Biofilms and infectious diseases: biology to mathematics and back again. FEMS Microbiol Lett. 322:1–7 - PubMed
-
- Cos P, Tote K, Horemans T, Maes L. 2010. Biofilms: an extra hurdle for effective antimicrobial therapy. Curr Pharm Des. 16:2279–2295 - PubMed
-
- Flemming H-C, Wingender J. 2010. The biofilm matrix. Nat Rev Microbiol. 8:623–633 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

