Building bridges: leveraging interdisciplinary collaborations in the development of biomaterials to meet clinical needs
- PMID: 22821772
- PMCID: PMC3706713
- DOI: 10.1002/adma.201201762
Building bridges: leveraging interdisciplinary collaborations in the development of biomaterials to meet clinical needs
Abstract
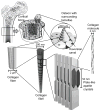
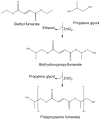
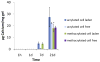
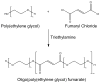
Our laboratory at Rice University has forged numerous collaborations with clinicians and basic scientists over the years to advance the development of novel biomaterials and the modification of existing materials to meet clinical needs. This review highlights collaborative advances in biomaterials research from our laboratory in the areas of scaffold development, drug delivery, and gene therapy, especially as related to applications in bone and cartilage tissue engineering.
Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures








References
-
- Myeroff C, Archdeacon M. J Bone Joint Surg Am. 2011;93:2227. - PubMed
-
- Sawin PD, Traynelis VC, Menezes aH. J Neurosurg. 1998;88:255. - PubMed
-
- Laurencin C, Khan Y, El-Amin SF. Expert Rev Med Devices. 2006;3:49. - PubMed
-
- Greenwald aS, Boden SD, Goldberg VM, Khan Y, Laurencin CT, Rosier RN. J Bone Joint Surg Am. 2001;83-A(Suppl):98. - PubMed
-
- Polak JM, Bishop AE. Ann N Y Acad Sci. 2006;1068:352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

