The effects of maternal exposure to bisphenol A on allergic lung inflammation into adulthood
- PMID: 22821851
- PMCID: PMC3621363
- DOI: 10.1093/toxsci/kfs227
The effects of maternal exposure to bisphenol A on allergic lung inflammation into adulthood
Abstract
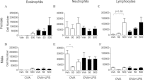
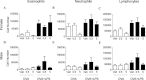
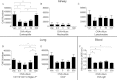
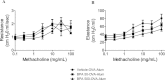
Bisphenol A (BPA) is a high-production volume chemical classified as an environmental estrogen and used primarily in the plastics industry. BPA's increased usage correlates with rising BPA levels in people and a corresponding increase in the incidence of asthma. Due to limited studies, the contribution of maternal BPA exposure to allergic asthma pathogenesis is unclear. Using two established mouse models of allergic asthma, we examined whether developmental exposure to BPA alters hallmarks of allergic lung inflammation in adult offspring. Pregnant C57BL/6 dams were gavaged with 0, 0.5, 5, 50, or 500 μg BPA/kg/day from gestational day 6 until postnatal day 21. To induce allergic inflammation, adult offspring were mucosally sensitized with inhaled ovalbumin containing low-dose lipopolysaccharide or ip sensitized using ovalbumin with alum followed by ovalbumin aerosol challenge. In the mucosal sensitization model, female offspring that were maternally exposed to ≥ 50 μg BPA/kg/day displayed enhanced airway lymphocytic and lung inflammation, compared with offspring of control dams. Peritoneally sensitized, female offspring exposed to ≤ 50 μg BPA/kg/day presented dampened lung eosinophilia, compared with vehicle controls. Male offspring did not exhibit these differences in either sensitization model. Our data demonstrate that maternal exposure to BPA has subtle and qualitatively different effects on allergic inflammation, which are critically dependent upon route of allergen sensitization and sex. However, these subtle, yet persistent changes due to developmental exposure to BPA did not lead to significant differences in overall airway responsiveness, suggesting that early life exposure to BPA does not exacerbate allergic inflammation into adulthood.
Figures



References
-
- Al-Hiyasat A. S., Darmani H., Elbetieha A. M. (2002). Effects of bisphenol A on adult male mouse fertility Eur. J. Oral Sci. 110 163–167 - PubMed
-
- Cannon J. M., Kostoryz E., Russo K. A., Smith R. E., Yourtee D. M. (2000). Bisphenol A and its biomaterial monomer derivatives alteration of in vitro cytochrome P450 metabolism in rat, minipig, and human. Biomacromolecules 1 656–664 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

