Autotaxin/ENPP2 regulates oligodendrocyte differentiation in vivo in the developing zebrafish hindbrain
- PMID: 22821873
- PMCID: PMC3422414
- DOI: 10.1002/glia.22381
Autotaxin/ENPP2 regulates oligodendrocyte differentiation in vivo in the developing zebrafish hindbrain
Abstract
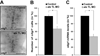
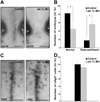
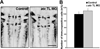
During development, progenitors that are committed to differentiate into oligodendrocytes, the myelinating cells of the central nervous system (CNS), are generated within discrete regions of the neuroepithelium. More specifically, within the developing spinal cord and hindbrain ventrally located progenitor cells that are characterized by the expression of the transcription factor olig2 give temporally rise to first motor neurons and then oligodendrocyte progenitors. The regulation of this temporal neuron-glial switch has been found complex and little is known about the extrinsic factors regulating it. Our studies described here identified a zebrafish ortholog to mammalian atx, which displays evolutionarily conserved expression pattern characteristics. Most interestingly, atx was found to be expressed by cells of the cephalic floor plate during a time period when ventrally-derived oligodendrocyte progenitors arise in the developing hindbrain of the zebrafish. Knock-down of atx expression resulted in a delay and/or inhibition of the timely appearance of oligodendrocyte progenitors and subsequent developmental stages of the oligodendrocyte lineage. This effect of atx knock-down was not accompanied by changes in the number of olig2-positive progenitor cells, the overall morphology of the axonal network or the number of somatic abducens motor neurons. Thus, our studies identified Atx as an extrinsic factor that is likely secreted by cells from the floor plate and that is involved in regulating specifically the progression of olig2-positive progenitor cells into lineage committed oligodendrocyte progenitors.
Copyright © 2012 Wiley Periodicals, Inc.
Figures


Similar articles
-
Olig2+ precursors produce abducens motor neurons and oligodendrocytes in the zebrafish hindbrain.J Neurosci. 2009 Feb 25;29(8):2322-33. doi: 10.1523/JNEUROSCI.3755-08.2009. J Neurosci. 2009. PMID: 19244509 Free PMC article.
-
Knockdown of Lingo1b protein promotes myelination and oligodendrocyte differentiation in zebrafish.Exp Neurol. 2014 Jan;251:72-83. doi: 10.1016/j.expneurol.2013.11.012. Epub 2013 Nov 18. Exp Neurol. 2014. PMID: 24262204
-
The Autotaxin-Lysophosphatidic Acid Axis Modulates Histone Acetylation and Gene Expression during Oligodendrocyte Differentiation.J Neurosci. 2015 Aug 12;35(32):11399-414. doi: 10.1523/JNEUROSCI.0345-15.2015. J Neurosci. 2015. PMID: 26269646 Free PMC article.
-
Regulation of oligodendrocyte development.Microsc Res Tech. 2001 Mar 15;52(6):740-5. doi: 10.1002/jemt.1058. Microsc Res Tech. 2001. PMID: 11276126 Review.
-
Olig gene function in CNS development and disease.Glia. 2006 Jul;54(1):1-10. doi: 10.1002/glia.20273. Glia. 2006. PMID: 16652341 Review.
Cited by
-
Pleotropic Roles of Autotaxin in the Nervous System Present Opportunities for the Development of Novel Therapeutics for Neurological Diseases.Mol Neurobiol. 2020 Jan;57(1):372-392. doi: 10.1007/s12035-019-01719-1. Epub 2019 Jul 30. Mol Neurobiol. 2020. PMID: 31364025 Review.
-
Lysophosphatidic Acid Signalling in Nervous System Development and Function.Neuromolecular Med. 2021 Mar;23(1):68-85. doi: 10.1007/s12017-020-08630-2. Epub 2020 Nov 5. Neuromolecular Med. 2021. PMID: 33151452 Free PMC article. Review.
-
Lysophosphatidic acid signalling in development.Development. 2015 Apr 15;142(8):1390-5. doi: 10.1242/dev.121723. Development. 2015. PMID: 25852197 Free PMC article. Review.
-
Differential Sox10 genomic occupancy in myelinating glia.Glia. 2015 Nov;63(11):1897-1914. doi: 10.1002/glia.22855. Epub 2015 May 14. Glia. 2015. PMID: 25974668 Free PMC article.
-
Recent advances in targeting the autotaxin-lysophosphatidate-lipid phosphate phosphatase axis in vivo.J Biomed Res. 2016 Jul;30(4):272-84. doi: 10.7555/JBR.30.20150058. Epub 2015 Aug 28. J Biomed Res. 2016. PMID: 27533936 Free PMC article. Review.
References
-
- Abramoff M, Magelhaes P, Ram S. Image Processing with image. Journal of Biophotonics Int. 2004;11:36–42.
-
- Bachner D, Ahrens M, Betat N, Schroder D, Gross G. Developmental expression analysis of murine autotaxin (ATX) Mech Dev. 1999;84:121–125. - PubMed
-
- Brosamle C, Halpern ME. Characterization of myelination in the developing zebrafish. Glia. 2002;39:47–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

