Calcium binding and allosteric signaling mechanisms for the sarcoplasmic reticulum Ca²+ ATPase
- PMID: 22821874
- PMCID: PMC3526986
- DOI: 10.1002/pro.2129
Calcium binding and allosteric signaling mechanisms for the sarcoplasmic reticulum Ca²+ ATPase
Abstract
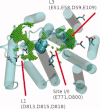
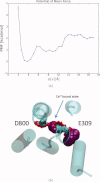
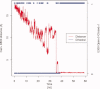
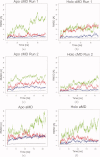
The sarcoplasmic reticulum Ca²⁺ ATPase (SERCA) is a membrane-bound pump that utilizes ATP to drive calcium ions from the myocyte cytosol against the higher calcium concentration in the sarcoplasmic reticulum. Conformational transitions associated with Ca²⁺-binding are important to its catalytic function. We have identified collective motions that partition SERCA crystallographic structures into multiple catalytically-distinct states using principal component analysis. Using Brownian dynamics simulations, we demonstrate the important contribution of surface-exposed, polar residues in the diffusional encounter of Ca²⁺. Molecular dynamics simulations indicate the role of Glu309 gating in binding Ca²⁺, as well as subsequent changes in the dynamics of SERCA's cytosolic domains. Together these data provide structural and dynamical insights into a multistep process involving Ca²⁺ binding and catalytic transitions.
Copyright © 2012 The Protein Society.
Figures





References
-
- Toyoshima C. Structural aspects of ion pumping by Ca2+-ATPase of sarcoplasmic reticulum. Arch Biochem Biophys. 2008;476:3–11. - PubMed
-
- Lewis S, Thomas D. Resolved conformational states of spin-labeled calcium-ATPase during the enzymic cycle. Biochemistry. 1992;31:7381–7389. - PubMed
-
- Toyoshima C, Inesi G. Structural basis of ion pumping by CA(2+)-ATpase of the sarcoplasmic reticulum. Annu Rev Biochem. 2004;73:269–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

