Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview
- PMID: 22821908
- PMCID: PMC3578601
- DOI: 10.1161/CIRCRESAHA.110.227512
Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview
Abstract
Since human embryonic stem cells were first differentiated to beating cardiomyocytes a decade ago, interest in their potential applications has increased exponentially. This has been further enhanced over recent years by the discovery of methods to induce pluripotency in somatic cells, including those derived from patients with hereditary cardiac diseases. Human pluripotent stem cells have been among the most challenging cell types to grow stably in culture, but advances in reagent development now mean that most laboratories can expand both embryonic and induced pluripotent stem cells robustly using commercially available products. However, differentiation protocols have lagged behind and in many cases only produce the cell types required with low efficiency. Cardiomyocyte differentiation techniques were also initially inefficient and not readily transferable across cell lines, but there are now a number of more robust protocols available. Here, we review the basic biology underlying the differentiation of pluripotent cells to cardiac lineages and describe current state-of-the-art protocols, as well as ongoing refinements. This should provide a useful entry for laboratories new to this area to start their research. Ultimately, efficient and reliable differentiation methodologies are essential to generate desired cardiac lineages to realize the full promise of human pluripotent stem cells for biomedical research, drug development, and clinical applications.
Figures
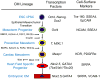


References
-
- Park IH, Lerou PH, Zhao R, Huo H, Daley GQ. Generation of human-induced pluripotent stem cells. Nat Protoc. 2008;3:1180–1186. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
-
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. - PubMed
-
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. - PubMed
-
- Carvajal-Vergara X, Sevilla A, D'Souza SL, Ang YS, Schaniel C, Lee DF, Yang L, Kaplan AD, Adler ED, Rozov R, Ge Y, Cohen N, Edelmann LJ, Chang B, Waghray A, Su J, Pardo S, Lichtenbelt KD, Tartaglia M, Gelb BD, Lemischka IR. Patient-specific induced pluripotent stem-cell-derived models of leopard syndrome. Nature. 2010;465:808–812. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

