Understanding cardiomyopathy phenotypes based on the functional impact of mutations in the myosin motor
- PMID: 22821910
- PMCID: PMC3947556
- DOI: 10.1161/CIRCRESAHA.110.223842
Understanding cardiomyopathy phenotypes based on the functional impact of mutations in the myosin motor
Abstract
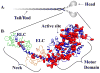
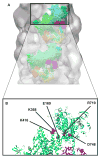
Hypertrophic (HCM) and dilated (DCM) cardiomyopathies are inherited diseases with a high incidence of death due to electric abnormalities or outflow tract obstruction. In many of the families afflicted with either disease, causative mutations have been identified in various sarcomeric proteins. In this review, we focus on mutations in the cardiac muscle molecular motor, myosin, and its associated light chains. Despite the >300 identified mutations, there is still no clear understanding of how these mutations within the same myosin molecule can lead to the dramatically different clinical phenotypes associated with HCM and DCM. Localizing mutations within myosin's molecular structure provides insight into the potential consequence of these perturbations to key functional domains of the motor. Review of biochemical and biophysical data that characterize the functional capacities of these mutant myosins suggests that mutant myosins with enhanced contractility lead to HCM, whereas those displaying reduced contractility lead to DCM. With gain and loss of function potentially being the primary consequence of a specific mutation, how these functional changes trigger the hypertrophic response and lead to the distinct HCM and DCM phenotypes will be the future investigative challenge.
Figures




References
-
- Olivotto I, Cecchi F, Poggesi C, Yacoub MH. Developmental origins of hypertrophic cardiomyopathy phenotypes: A unifying hypothesis. Nat Rev Cardiol. 2009;6:317–321. - PubMed
-
- Liew CC, Dzau VJ. Molecular genetics and genomics of heart failure. Nat Rev Genet. 2004;5:811–825. - PubMed
-
- Ramaraj R. Hypertrophic cardiomyopathy: Etiology, diagnosis, and treatment. Cardiol Rev. 2008;16:172–180. - PubMed
-
- Kamisago M, Sharma SD, DePalma SR, Solomon S, Sharma P, McDonough B, Smoot L, Mullen MP, Woolf PK, Wigle ED, Seidman JG, Seidman CE. Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy. N Engl J Med. 2000;343:1688–1696. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

