Treatment of intracranial aneurysms using the pipeline flow-diverter embolization device: a single-center experience with long-term follow-up results
- PMID: 22821921
- PMCID: PMC7966552
- DOI: 10.3174/ajnr.A3246
Treatment of intracranial aneurysms using the pipeline flow-diverter embolization device: a single-center experience with long-term follow-up results
Abstract
Background and purpose: Flow-diverting devices now offer a new treatment alternative for cerebral aneurysms. We present the results of a large single-center series of patients treated with the PED, including long-term follow-up.
Materials and methods: Between November 2008 and September 2011, sidewall aneurysms with a wide neck (≥4 mm) or unfavorable dome-neck ratio (≤1.5); large/giant, fusiform, dissecting, blister-like, and recurrent sidewall aneurysms; aneurysms at difficult angles; and aneurysms in which a branch was originating directly from the sac were treated with the PED. Patients were premedicated with dual antiplatelet medications. Data, including demographics, aneurysm features, clinical presentation, complications, results, and follow-up information, for up to 2 years are presented.
Results: Two hundred fifty-one aneurysms in 191 patients were treated. Of these, 96 (38.3%) were large or giant (≥10 mm). In 34/251 (13.5%), PEDs were used for retreatment. Adjunctive coiling was performed in 11 aneurysms (2.1%). The mean number of devices per aneurysm was 1.3. One aneurysm ruptured in the fourth month posttreatment (0.5%), and symptomatic in-construct stenosis was detected in 1 patient (0.5%) treated with percutaneous transarterial angioplasty. Any event rate was 27/191 (14.1%), with a permanent morbidity of 1% and mortality of 0.5%. Control angiography was available in 182 (95.3%) patients with 239 (95.2%) aneurysms. In 121 aneurysms (48.2%), 1- to 2-year control angiography was available. The aneurysm occlusion rate was 91.2% in 6 months, increasing to 94.6%.
Conclusions: Use of the PED is safe, efficacious, and durable in cerebral aneurysm treatment, with low morbidity-mortality and high occlusion rates as confirmed with mid- to long-term control angiography.
Figures
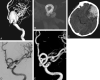

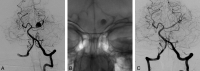
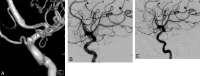


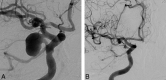
References
-
- Molyneux A, Kerr R, Stratton I, et al. , for the International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002; 360: 1267– 74 - PubMed
-
- McDougall CG, Spetzler RF, Zabramski JM, et al. . The Barrow Ruptured Aneurysm Trial. J Neurosurg 2012; 116: 135– 44 - PubMed
-
- Lylyk P, Ferrario A, Pasbon B, et al. . Buenos Aires experience with the Neuroform self-expanding stent for the treatment of intracranial aneurysms. J Neurosurg 2005; 102: 235– 41 - PubMed
-
- Jahromi BS, Mocco J, Bang JA, et al. . Clinical and angiographic outcome after endovascular management of giant intracranial aneurysms. Neurosurgery 2008; 63: 662– 74, discussion 674–75 - PubMed
-
- Mocco J, Snyder KV, Albuquerque FC, et al. . Treatment of intracranial aneurysms with the Enterprise stent: a multicenter registry. J Neurosurg 2009; 110: 35– 39 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
