CD8+CD45RA+CCR7+FOXP3+ T cells with immunosuppressive properties: a novel subset of inducible human regulatory T cells
- PMID: 22821963
- PMCID: PMC3424334
- DOI: 10.4049/jimmunol.1200122
CD8+CD45RA+CCR7+FOXP3+ T cells with immunosuppressive properties: a novel subset of inducible human regulatory T cells
Abstract
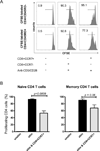
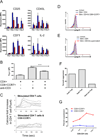
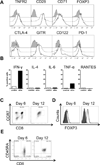
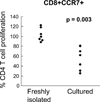
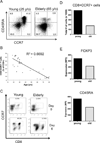
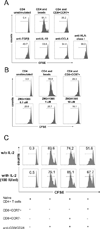
CD8 T cells stimulated with a suboptimal dose of anti-CD3 Abs (100 pg/ml) in the presence of IL-15 retain a naive phenotype with expression of CD45RA, CD28, CD27, and CCR7 but acquire new functions and differentiate into immunosuppressive T cells. CD8+CCR7+ regulatory T cells (Tregs) express FOXP3 and prevent CD4 T cells from responding to TCR stimulation and entering the cell cycle. Naive CD4 T cells are more susceptible to inhibition than memory cells. The suppressive activity of CD8+CCR7+ Tregs is not mediated by IL-10, TGF-β, CTLA-4, CCL4, or adenosine and relies on interference with very early steps of the TCR signaling cascade. Specifically, CD8+CCR7+ Tregs prevent TCR-induced phosphorylation of ZAP70 and dampen the rise of intracellular calcium in CD4 T cells. The inducibility of CD8+CCR7+ Tregs is correlated with the age of the individual with PBLs of donors older than 60 y yielding low numbers of FOXP3(low) CD8 Tregs. Loss of CD8+CCR7+ Tregs in the elderly host may be of relevance in the aging immune system as immunosenescence is associated with a state of chronic smoldering inflammation.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures


References
-
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. - PubMed
-
- Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions (*) Annu Rev Immunol. 2009;27:551–589. - PubMed
-
- Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. - PubMed
-
- Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. - PubMed
-
- Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR 42527/AR/NIAMS NIH HHS/United States
- EY 11916/EY/NEI NIH HHS/United States
- AI 90019/AI/NIAID NIH HHS/United States
- R01 AI044142/AI/NIAID NIH HHS/United States
- P01 HL058000/HL/NHLBI NIH HHS/United States
- U19 AI090019/AI/NIAID NIH HHS/United States
- HL 58000/HL/NHLBI NIH HHS/United States
- R56 AI044142/AI/NIAID NIH HHS/United States
- R01 EY011916/EY/NEI NIH HHS/United States
- AI 57266/AI/NIAID NIH HHS/United States
- AI 44142/AI/NIAID NIH HHS/United States
- U19 AI057266/AI/NIAID NIH HHS/United States
- R01 AR042527/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

