Hfq influences multiple transport systems and virulence in the plant pathogen Agrobacterium tumefaciens
- PMID: 22821981
- PMCID: PMC3457239
- DOI: 10.1128/JB.00510-12
Hfq influences multiple transport systems and virulence in the plant pathogen Agrobacterium tumefaciens
Abstract
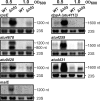
The Hfq protein mediates gene regulation by small RNAs (sRNAs) in about 50% of all bacteria. Depending on the species, phenotypic defects of an hfq mutant range from mild to severe. Here, we document that the purified Hfq protein of the plant pathogen and natural genetic engineer Agrobacterium tumefaciens binds to the previously described sRNA AbcR1 and its target mRNA atu2422, which codes for the substrate binding protein of an ABC transporter taking up proline and γ-aminobutyric acid (GABA). Several other ABC transporter components were overproduced in an hfq mutant compared to their levels in the parental strain, suggesting that Hfq plays a major role in controlling the uptake systems and metabolic versatility of A. tumefaciens. The hfq mutant showed delayed growth, altered cell morphology, and reduced motility. Although the DNA-transferring type IV secretion system was produced, tumor formation by the mutant strain was attenuated, demonstrating an important contribution of Hfq to plant transformation by A. tumefaciens.
Figures






Similar articles
-
The RNase YbeY Is Vital for Ribosome Maturation, Stress Resistance, and Virulence of the Natural Genetic Engineer Agrobacterium tumefaciens.J Bacteriol. 2019 May 8;201(11):e00730-18. doi: 10.1128/JB.00730-18. Print 2019 Jun 1. J Bacteriol. 2019. PMID: 30885931 Free PMC article.
-
Profound impact of Hfq on nutrient acquisition, metabolism and motility in the plant pathogen Agrobacterium tumefaciens.PLoS One. 2014 Oct 17;9(10):e110427. doi: 10.1371/journal.pone.0110427. eCollection 2014. PLoS One. 2014. PMID: 25330313 Free PMC article.
-
Small RNA-mediated control of the Agrobacterium tumefaciens GABA binding protein.Mol Microbiol. 2011 Apr;80(2):492-506. doi: 10.1111/j.1365-2958.2011.07589.x. Epub 2011 Mar 3. Mol Microbiol. 2011. PMID: 21320185
-
Small Noncoding RNAs in Agrobacterium tumefaciens.Curr Top Microbiol Immunol. 2018;418:195-213. doi: 10.1007/82_2018_84. Curr Top Microbiol Immunol. 2018. PMID: 29556823 Review.
-
Bacterial Small Regulatory RNAs and Hfq Protein.Biochemistry (Mosc). 2015 Dec;80(13):1647-54. doi: 10.1134/S0006297915130027. Biochemistry (Mosc). 2015. PMID: 26878571 Review.
Cited by
-
Arginine-Rich Small Proteins with a Domain of Unknown Function, DUF1127, Play a Role in Phosphate and Carbon Metabolism of Agrobacterium tumefaciens.J Bacteriol. 2020 Oct 22;202(22):e00309-20. doi: 10.1128/JB.00309-20. Print 2020 Oct 22. J Bacteriol. 2020. PMID: 33093235 Free PMC article.
-
Edwardsiella tarda Hfq: impact on host infection and global protein expression.Vet Res. 2014 Feb 25;45(1):23. doi: 10.1186/1297-9716-45-23. Vet Res. 2014. PMID: 24568370 Free PMC article.
-
A genetic screen to identify factors affected by undecaprenyl phosphate recycling uncovers novel connections to morphogenesis in Escherichia coli.Mol Microbiol. 2021 Feb;115(2):191-207. doi: 10.1111/mmi.14609. Epub 2020 Oct 12. Mol Microbiol. 2021. PMID: 32979869 Free PMC article.
-
sRNA-Mediated Regulation of P-Fimbriae Phase Variation in Uropathogenic Escherichia coli.PLoS Pathog. 2015 Aug 20;11(8):e1005109. doi: 10.1371/journal.ppat.1005109. eCollection 2015 Aug. PLoS Pathog. 2015. PMID: 26291711 Free PMC article.
-
Orchestration of virulence factor expression and modulation of biofilm dispersal in Erwinia amylovora through activation of the Hfq-dependent small RNA RprA.Mol Plant Pathol. 2021 Feb;22(2):255-270. doi: 10.1111/mpp.13024. Epub 2020 Dec 13. Mol Plant Pathol. 2021. PMID: 33314618 Free PMC article.
References
-
- Aiba H. 2007. Mechanism of RNA silencing by Hfq-binding small RNAs. Curr. Opin. Microbiol. 10:134–139 - PubMed
-
- Aiba H, Adhya S, de Crombrugghe B. 1981. Evidence for two functional gal promoters in intact Escherichia coli cells. J. Biol. Chem. 256:11905–11910 - PubMed
-
- Berghoff BA, Glaeser J, Sharma CM, Vogel J, Klug G. 2009. Photooxidative stress-induced and abundant small RNAs in Rhodobacter sphaeroides. Mol. Microbiol. 74:1497–1512 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

