Dual targeting of mTOR and aurora-A kinase for the treatment of uterine Leiomyosarcoma
- PMID: 22821997
- PMCID: PMC3432751
- DOI: 10.1158/1078-0432.CCR-12-0436
Dual targeting of mTOR and aurora-A kinase for the treatment of uterine Leiomyosarcoma
Abstract
Purpose: The significance of mTOR activation in uterine leiomyosarcoma (ULMS) and its potential as a therapeutic target were investigated. Furthermore, given that effective therapies likely require combination mTOR blockade with inhibition of other targets, coupled with recent observations suggesting that Aurora-A kinase (Aurk-A) deregulations commonly occur in ULMS, the preclinical impact of dually targeting both pathways was evaluated.
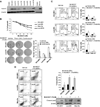
Experimental design: Immunohistochemical staining was used to evaluate expression of activated mTOR components in a large (>200 samples) ULMS tissue microarray. Effects of mTOR blockade (using rapamycin) and Aurk-A inhibition (using MLN8237) alone and in combination on human ULMS cell growth, cell-cycle progression, and apoptosis were assessed in cellular assays. Drug interactions were determined via combination index analyses. The antitumor effects of inhibitors alone or in combination were evaluated in vivo.
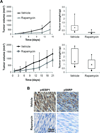
Results: Enhanced mTOR activation was seen in human ULMS samples. Increased pS6RP and p4EBP1 expression correlated with disease progression; p4EBP1 was found to be an independent prognosticator of patient outcome. Rapamycin inhibited growth and cell-cycle progression of ULMS cell strains/lines in culture. However, only a cytostatic effect on tumor growth was found in vivo. Combining rapamycin with MLN8237 profoundly (and synergistically) abrogated ULMS cells' growth in culture; interestingly, these effects were seen only when MLN8237 was preadministered. This novel therapeutic combination and scheduling regimen resulted in marked tumor growth inhibition in vivo.
Conclusions: mTOR and Aurk-A pathways are commonly deregulated in ULMS. Preclinical data support further exploration of dual mTOR and Aurk-A therapeutic blockade for human ULMS.
©2012 AACR.
Conflict of interest statement
Figures




References
-
- Schwartz LB, Diamond MP, Schwartz PE. Leiomyosarcomas: clinical presentation. Am J Obstet Gynecol. 1993;168:180–183. - PubMed
-
- Smith DC, Macdonald OK, Lee CM, Gaffney DK. Survival impact of lymph node dissection in endometrial adenocarcinoma: a surveillance, epidemiology, and end results analysis. Int J Gynecol Cancer. 2008;18:255–261. - PubMed
-
- Zivanovic O, Leitao MM, Iasonos A, Jacks LM, Zhou Q, Abu-Rustum NR, et al. Stage-specific outcomes of patients with uterine leiomyosarcoma: a comparison of the international Federation of gynecology and obstetrics and american joint committee on cancer staging systems. J Clin Oncol. 2009;27:2066–2072. - PMC - PubMed
-
- Salazar OM, Bonfiglio TA, Patten SF, Keller BE, Feldstein M, Dunne ME, et al. Uterine sarcomas: natural history, treatment and prognosis. Cancer. 1978;42:1152–1160. - PubMed
-
- Bodner K, Bodner-Adler B, Kimberger O, Czerwenka K, Leodolter S, Mayerhofer K. Evaluating prognostic parameters in women with uterine leiomyosarcoma. A clinicopathologic study. J Reprod Med. 2003;48:95–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

