Carbon monoxide induces cardiac arrhythmia via induction of the late Na+ current
- PMID: 22822026
- PMCID: PMC3622900
- DOI: 10.1164/rccm.201204-0688OC
Carbon monoxide induces cardiac arrhythmia via induction of the late Na+ current
Abstract
Rationale: Clinical reports describe life-threatening cardiac arrhythmias after environmental exposure to carbon monoxide (CO) or accidental CO poisoning. Numerous case studies describe disruption of repolarization and prolongation of the QT interval, yet the mechanisms underlying CO-induced arrhythmias are unknown.
Objectives: To understand the cellular basis of CO-induced arrhythmias and to identify an effective therapeutic approach.
Methods: Patch-clamp electrophysiology and confocal Ca(2+) and nitric oxide (NO) imaging in isolated ventricular myocytes was performed together with protein S-nitrosylation to investigate the effects of CO at the cellular and molecular levels, whereas telemetry was used to investigate effects of CO on electrocardiogram recordings in vivo.
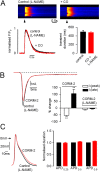
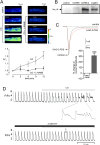
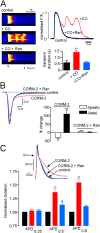
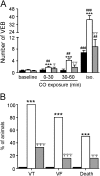
Measurements and main results: CO increased the sustained (late) component of the inward Na(+) current, resulting in prolongation of the action potential and the associated intracellular Ca(2+) transient. In more than 50% of myocytes these changes progressed to early after-depolarization-like arrhythmias. CO elevated NO levels in myocytes and caused S-nitrosylation of the Na(+) channel, Na(v)1.5. All proarrhythmic effects of CO were abolished by the NO synthase inhibitor l-NAME, and reversed by ranolazine, an inhibitor of the late Na(+) current. Ranolazine also corrected QT variability and arrhythmias induced by CO in vivo, as monitored by telemetry.
Conclusions: Our data indicate that the proarrhythmic effects of CO arise from activation of NO synthase, leading to NO-mediated nitrosylation of Na(V)1.5 and to induction of the late Na(+) current. We also show that the antianginal drug ranolazine can abolish CO-induced early after-depolarizations, highlighting a novel approach to the treatment of CO-induced arrhythmias.
Figures



Comment in
-
The paradox of carbon monoxide and the heart.Am J Respir Crit Care Med. 2012 Oct 1;186(7):582-3. doi: 10.1164/rccm.201207-1341ED. Am J Respir Crit Care Med. 2012. PMID: 23027850 No abstract available.
Similar articles
-
A novel, potent, and selective inhibitor of cardiac late sodium current suppresses experimental arrhythmias.J Pharmacol Exp Ther. 2013 Jan;344(1):23-32. doi: 10.1124/jpet.112.198887. Epub 2012 Sep 25. J Pharmacol Exp Ther. 2013. PMID: 23010360
-
Cellular basis for the electrocardiographic and arrhythmic manifestations of Timothy syndrome: effects of ranolazine.Heart Rhythm. 2007 May;4(5):638-47. doi: 10.1016/j.hrthm.2006.12.046. Epub 2007 Jan 7. Heart Rhythm. 2007. PMID: 17467634 Free PMC article.
-
The paradox of carbon monoxide and the heart.Am J Respir Crit Care Med. 2012 Oct 1;186(7):582-3. doi: 10.1164/rccm.201207-1341ED. Am J Respir Crit Care Med. 2012. PMID: 23027850 No abstract available.
-
New treatment options for late Na current, arrhythmias, and diastolic dysfunction.Curr Heart Fail Rep. 2012 Sep;9(3):183-91. doi: 10.1007/s11897-012-0099-3. Curr Heart Fail Rep. 2012. PMID: 22767404 Free PMC article. Review.
-
Electrophysiologic properties and antiarrhythmic actions of a novel antianginal agent.J Cardiovasc Pharmacol Ther. 2004 Sep;9 Suppl 1:S65-83. doi: 10.1177/107424840400900106. J Cardiovasc Pharmacol Ther. 2004. PMID: 15378132 Review.
Cited by
-
Carbon monoxide activates large-conductance calcium-activated potassium channels of human cardiac fibroblasts through various mechanisms.Korean J Physiol Pharmacol. 2021 May 1;25(3):227-237. doi: 10.4196/kjpp.2021.25.3.227. Korean J Physiol Pharmacol. 2021. PMID: 33859063 Free PMC article.
-
Resveratrol protects rabbit ventricular myocytes against oxidative stress-induced arrhythmogenic activity and Ca2+ overload.Acta Pharmacol Sin. 2013 Sep;34(9):1164-73. doi: 10.1038/aps.2013.82. Epub 2013 Aug 5. Acta Pharmacol Sin. 2013. PMID: 23912472 Free PMC article.
-
Modulation of ion channels and transporters by carbon monoxide: causes for concern?Front Physiol. 2012 Dec 20;3:477. doi: 10.3389/fphys.2012.00477. eCollection 2012. Front Physiol. 2012. PMID: 23267333 Free PMC article. No abstract available.
-
Effectiveness of Hyperbaric Oxygenation Versus Normobaric Oxygenation Therapy in Carbon Monoxide Poisoning: A Systematic Review.Cureus. 2019 Oct 15;11(10):e5916. doi: 10.7759/cureus.5916. Cureus. 2019. PMID: 31788375 Free PMC article. Review.
-
Carbon monoxide releasing molecule-2 suppresses stretch-activated atrial natriuretic peptide secretion by activating large-conductance calcium-activated potassium channels.Korean J Physiol Pharmacol. 2022 Mar 1;26(2):125-133. doi: 10.4196/kjpp.2022.26.2.125. Korean J Physiol Pharmacol. 2022. PMID: 35203062 Free PMC article.
References
-
- Von Burg R. Carbon monoxide. J Appl Toxicol 1999;19:379–386 - PubMed
-
- Gandini C, Castoldi AF, Candura SM, Locatelli C, Butera R, Priori S, Manzo L. Carbon monoxide cardiotoxicity. J Toxicol Clin Toxicol 2001;39:35–44 - PubMed
-
- Henry CR, Satran D, Lindgren B, Adkinson C, Nicholson CI, Henry TD. Myocardial injury and long-term mortality following moderate to severe carbon monoxide poisoning. JAMA 2006;295:398–402 - PubMed
-
- Henry TD, Lesser JR, Satran D. Myocardial fibrosis from severe carbon monoxide poisoning detected by cardiac magnetic resonance imaging. Circulation 2008;118:792. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

