CD11c(+)/CD11b(+) cells are critical for organic dust-elicited murine lung inflammation
- PMID: 22822029
- PMCID: PMC3547108
- DOI: 10.1165/rcmb.2012-0095OC
CD11c(+)/CD11b(+) cells are critical for organic dust-elicited murine lung inflammation
Abstract
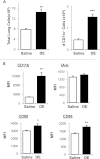
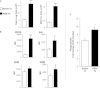
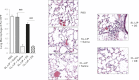
Organic dust exposure in the agricultural industry results in significant lung disease. Macrophage infiltrates are increased in the lungs after organic dust exposures, yet the phenotype and functional importance of these cells remain unclear. Using an established intranasal inhalation murine model of dust-induced lung inflammation, animals were treated once or daily for 3 weeks with swine confinement organic dust extract (DE). Repetitive DE treatment for 3 weeks resulted in significant increases in CD11c(+)/CD11b(+) macrophages in whole lung-associated tissue. These cells displayed increased costimulatory molecule (CD80 and CD86) expression, enhanced phagocytic ability, and an increased production of IL-6, CXCL1, and CXCL2. Similar findings were observed with the CD11c(+)/CD11b(+) macrophage infiltrate after repetitive exposure to peptidoglycan, a major DE component. To determine the functional importance of macrophages in mediating DE-induced airway inflammation, lung macrophages were selectively depleted using a well-established intranasal clodronate liposome depletion/suicide strategy. First, macrophage depletion by clodronate liposomes resulted in significant reductions in airway neutrophil influx and TNF-α and IL-6 production after a single exposure to DE. In contrast, after repetitive 3-week exposure to DE, airway lavage fluid and lung tissue neutrophils were significantly increased in clodronate liposome-treated mice compared with control mice. A histological examination of lung tissue demonstrated striking increases in alveolar and bronchiolar inflammation, as well as in the size and distribution of cellular aggregates in clodronate-liposome versus saline-liposome groups repetitively exposed to DE. These studies demonstrate that DE elicits activated CD11c(+)/CD11b(+) macrophages in the lung, which play a critical role in regulating the outcome of DE-induced airway inflammation.
Figures




Similar articles
-
Toll-like receptor 2 regulates organic dust-induced airway inflammation.Am J Respir Cell Mol Biol. 2011 Oct;45(4):711-9. doi: 10.1165/rcmb.2010-0427OC. Epub 2011 Jan 28. Am J Respir Cell Mol Biol. 2011. PMID: 21278324 Free PMC article.
-
Intranasal organic dust exposure-induced airway adaptation response marked by persistent lung inflammation and pathology in mice.Am J Physiol Lung Cell Mol Physiol. 2009 Jun;296(6):L1085-95. doi: 10.1152/ajplung.90622.2008. Epub 2009 Apr 24. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19395665 Free PMC article.
-
αβ T cells and a mixed Th1/Th17 response are important in organic dust-induced airway disease.Ann Allergy Asthma Immunol. 2012 Oct;109(4):266-273.e2. doi: 10.1016/j.anai.2012.06.015. Epub 2012 Jul 24. Ann Allergy Asthma Immunol. 2012. PMID: 23010233 Free PMC article.
-
The guardians of pulmonary harmony: alveolar macrophages orchestrating the symphony of lung inflammation and tissue homeostasis.Eur Respir Rev. 2024 May 29;33(172):230263. doi: 10.1183/16000617.0263-2023. Print 2024 Apr 30. Eur Respir Rev. 2024. PMID: 38811033 Free PMC article. Review.
-
Does tissue imprinting restrict macrophage plasticity?Nat Immunol. 2021 Feb;22(2):118-127. doi: 10.1038/s41590-020-00849-2. Epub 2021 Jan 18. Nat Immunol. 2021. PMID: 33462453 Review.
Cited by
-
Organic dust-induced lung injury and repair: Bi-directional regulation by TNFα and IL-10.J Immunotoxicol. 2020 Dec;17(1):153-162. doi: 10.1080/1547691X.2020.1776428. J Immunotoxicol. 2020. PMID: 32634062 Free PMC article.
-
Role of Macrophages in Acute Lung Injury and Chronic Fibrosis Induced by Pulmonary Toxicants.Toxicol Sci. 2019 Apr 1;168(2):287-301. doi: 10.1093/toxsci/kfy309. Toxicol Sci. 2019. PMID: 30590802 Free PMC article. Review.
-
Occupational agriculture organic dust exposure and its relationship to asthma and airway inflammation in adults.J Asthma. 2016 Jun;53(5):471-7. doi: 10.3109/02770903.2015.1116089. Epub 2016 Jan 19. J Asthma. 2016. PMID: 26785925 Free PMC article. Review.
-
Age Impacts Pulmonary Inflammation and Systemic Bone Response to Inhaled Organic Dust Exposure.J Toxicol Environ Health A. 2015;78(19):1201-16. doi: 10.1080/15287394.2015.1075165. Epub 2015 Oct 5. J Toxicol Environ Health A. 2015. PMID: 26436836 Free PMC article.
-
Pattern recognition scavenger receptor A/CD204 regulates airway inflammatory homeostasis following organic dust extract exposures.J Immunotoxicol. 2015 Jan-Mar;12(1):64-73. doi: 10.3109/1547691X.2014.882449. Epub 2014 Feb 3. J Immunotoxicol. 2015. PMID: 24491035 Free PMC article.
References
-
- Von Essen S, Romberger D. The respiratory inflammatory response to the swine confinement building environment: the adaptation to respiratory exposures in the chronically exposed worker. J Agric Saf Health 2003;9:185–196 - PubMed
-
- Eduard W, Pearce N, Douwes J. Chronic bronchitis, COPD, and lung function in farmers: the role of biological agents. Chest 2009;136:716–725 - PubMed
-
- Sundblad BM, von Scheele I, Palmberg L, Olsson M, Larsson K. Repeated exposure to organic material alters inflammatory and physiological airway responses. Eur Respir J 2009;34:80–88 - PubMed
-
- Palmberg L, Larssson BM, Malmberg P, Larsson K. Airway responses of healthy farmers and nonfarmers to exposure in a swine confinement building. Scand J Work Environ Health 2002;28:256–263 - PubMed
-
- Schwartz DA, Donham KJ, Olenchock SA, Popendorf WJ, Van Fossen DS, Burmeister LF, Merchant JA. Determinants of longitudinal changes in spirometric function among swine confinement operators and farmers. Am J Respir Crit Care Med 1995;151:47–53 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

