The tissue-type plasminogen activator-plasminogen activator inhibitor 1 complex promotes neurovascular injury in brain trauma: evidence from mice and humans
- PMID: 22822039
- PMCID: PMC3501968
- DOI: 10.1093/brain/aws178
The tissue-type plasminogen activator-plasminogen activator inhibitor 1 complex promotes neurovascular injury in brain trauma: evidence from mice and humans
Abstract
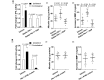
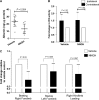
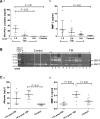
The neurovascular unit provides a dynamic interface between the circulation and central nervous system. Disruption of neurovascular integrity occurs in numerous brain pathologies including neurotrauma and ischaemic stroke. Tissue plasminogen activator is a serine protease that converts plasminogen to plasmin, a protease that dissolves blood clots. Besides its role in fibrinolysis, tissue plasminogen activator is abundantly expressed in the brain where it mediates extracellular proteolysis. However, proteolytically active tissue plasminogen activator also promotes neurovascular disruption after ischaemic stroke; the molecular mechanisms of this process are still unclear. Tissue plasminogen activator is naturally inhibited by serine protease inhibitors (serpins): plasminogen activator inhibitor-1, neuroserpin or protease nexin-1 that results in the formation of serpin:protease complexes. Proteases and serpin:protease complexes are cleared through high-affinity binding to low-density lipoprotein receptors, but their binding to these receptors can also transmit extracellular signals across the plasma membrane. The matrix metalloproteinases are the second major proteolytic system in the mammalian brain, and like tissue plasminogen activators are pivotal to neurological function but can also degrade structures of the neurovascular unit after injury. Herein, we show that tissue plasminogen activator potentiates neurovascular damage in a dose-dependent manner in a mouse model of neurotrauma. Surprisingly, inhibition of activity following administration of plasminogen activator inhibitor-1 significantly increased cerebrovascular permeability. This led to our finding that formation of complexes between tissue plasminogen activator and plasminogen activator inhibitor-1 in the brain parenchyma facilitates post-traumatic cerebrovascular damage. We demonstrate that following trauma, the complex binds to low-density lipoprotein receptors, triggering the induction of matrix metalloproteinase-3. Accordingly, pharmacological inhibition of matrix metalloproteinase-3 attenuates neurovascular permeability and improves neurological function in injured mice. Our results are clinically relevant, because concentrations of tissue plasminogen activator: plasminogen activator inhibitor-1 complex and matrix metalloproteinase-3 are significantly elevated in cerebrospinal fluid of trauma patients and correlate with neurological outcome. In a separate study, we found that matrix metalloproteinase-3 and albumin, a marker of cerebrovascular damage, were significantly increased in brain tissue of patients with neurotrauma. Perturbation of neurovascular homeostasis causing oedema, inflammation and cell death is an important cause of acute and long-term neurological dysfunction after trauma. A role for the tissue plasminogen activator-matrix metalloproteinase axis in promoting neurovascular disruption after neurotrauma has not been described thus far. Targeting tissue plasminogen activator: plasminogen activator inhibitor-1 complex signalling or downstream matrix metalloproteinase-3 induction may provide viable therapeutic strategies to reduce cerebrovascular permeability after neurotrauma.
Figures


References
-
- Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta. 2011;1812:252–64. - PubMed
-
- Armstead WM, Nassar T, Akkawi S, Smith DH, Chen XH, Cines DB, et al. Neutralizing the neurotoxic effects of exogenous and endogenous tPA. Nat Neurosci. 2006;9:1150–5. - PubMed
-
- Dietzmann K, von Bossanyi P, Krause D, Wittig H, Mawrin C, Kirches E. Expression of the plasminogen activator system and the inhibitors PAI-1 and PAI-2 in posttraumatic lesions of the CNS and brain injuries following dramatic circulatory arrests: an immunohistochemical study. Pathol Res Prac. 2000;196:15–21. - PubMed

