Induction of oxazolone-mediated features of atopic dermatitis in NOD-scid IL2Rγ(null) mice engrafted with human peripheral blood mononuclear cells
- PMID: 22822046
- PMCID: PMC3529345
- DOI: 10.1242/dmm.009167
Induction of oxazolone-mediated features of atopic dermatitis in NOD-scid IL2Rγ(null) mice engrafted with human peripheral blood mononuclear cells
Abstract
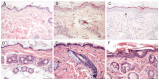
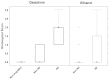
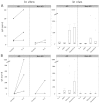
Animal models mimicking human diseases have been used extensively to study the pathogenesis of autoimmune diseases and the efficacy of potential therapeutics. They are, however, limited with regard to their similarity to the human disease and cannot be used if the antagonist and its cognate receptor require high similarity in structure or binding. Here, we examine the induction of oxazolone-mediated features of atopic dermatitis (AD) in NOD-scid IL2Rγ(null) mice engrafted with human peripheral blood mononuclear cells (PBMC). The mice developed the same symptoms as immunocompetent BALB/c mice. Histological alterations induced by oxazolone were characterized by keratosis, epithelial hyperplasia and influx of inflammatory cells into the dermis and epidermis. The cellular infiltrate was identified as human leukocytes, with T cells being the major constituent. In addition, oxazolone increased human serum IgE levels. The response, however, required the engraftment of PBMC derived from patients suffering from AD, which suggests that this model reflects the immunological status of the donor. Taken together, the model described here has the potential to evaluate the efficacy of therapeutics targeting human lymphocytes in vivo and, in addition, might be developed further to elucidate molecular mechanisms inducing and sustaining flares of the disease.
Figures


Similar articles
-
Oxazolone and ethanol induce colitis in non-obese diabetic-severe combined immunodeficiency interleukin-2Rγ(null) mice engrafted with human peripheral blood mononuclear cells.Clin Exp Immunol. 2013 May;172(2):349-62. doi: 10.1111/cei.12057. Clin Exp Immunol. 2013. PMID: 23574330 Free PMC article.
-
A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene.Clin Immunol. 2008 Mar;126(3):303-14. doi: 10.1016/j.clim.2007.11.001. Epub 2007 Dec 21. Clin Immunol. 2008. PMID: 18096436
-
NOD-scid IL2R γnull mice engrafted with human peripheral blood mononuclear cells as a model to test therapeutics targeting human signaling pathways.J Transl Med. 2013 Jan 7;11:4. doi: 10.1186/1479-5876-11-4. J Transl Med. 2013. PMID: 23294516 Free PMC article.
-
NOD/Shi-scid IL2rgamma(null) (NOG) mice more appropriate for humanized mouse models.Curr Top Microbiol Immunol. 2008;324:53-76. doi: 10.1007/978-3-540-75647-7_3. Curr Top Microbiol Immunol. 2008. PMID: 18481452 Review.
-
Humanized SCID mouse models for biomedical research.Curr Top Microbiol Immunol. 2008;324:25-51. doi: 10.1007/978-3-540-75647-7_2. Curr Top Microbiol Immunol. 2008. PMID: 18481451 Review.
Cited by
-
Effect of a Product Containing Xyloglucan and Pea Protein on a Murine Model of Atopic Dermatitis.Int J Mol Sci. 2020 May 19;21(10):3596. doi: 10.3390/ijms21103596. Int J Mol Sci. 2020. PMID: 32438777 Free PMC article.
-
A mouse model for ulcerative colitis based on NOD-scid IL2R γnull mice reconstituted with peripheral blood mononuclear cells from affected individuals.Dis Model Mech. 2016 Sep 1;9(9):985-97. doi: 10.1242/dmm.025452. Epub 2016 Aug 4. Dis Model Mech. 2016. PMID: 27491073 Free PMC article.
-
Development of novel elastic vesicle-based topical formulation of cetirizine dihydrochloride for treatment of atopic dermatitis.AAPS PharmSciTech. 2013 Dec;14(4):1284-93. doi: 10.1208/s12249-013-0017-3. AAPS PharmSciTech. 2013. PMID: 23959702 Free PMC article.
-
Dual Delivery of Fluticasone Propionate and Levocetirizine Dihydrochloride for the Management of Atopic Dermatitis Using a Microemulsion-Based Topical Gel.ACS Omega. 2022 Feb 28;7(9):7696-7705. doi: 10.1021/acsomega.1c06393. eCollection 2022 Mar 8. ACS Omega. 2022. PMID: 35284709 Free PMC article.
-
Effects of the Fruit Extract of Tribulus terrestris on Skin Inflammation in Mice with Oxazolone-Induced Atopic Dermatitis through Regulation of Calcium Channels, Orai-1 and TRPV3, and Mast Cell Activation.Evid Based Complement Alternat Med. 2017;2017:8312946. doi: 10.1155/2017/8312946. Epub 2017 Nov 14. Evid Based Complement Alternat Med. 2017. PMID: 29348776 Free PMC article.
References
-
- Biedermann T., Zimmermann S., Himmelrich H., Gumy A., Egeter O., Sakrauski A. K., Seegmüller I., Voigt H., Launois P., Levine A. D., et al. (2001). IL-4 instructs TH1 responses and resistance to Leishmania major in susceptible BALB/c mice. Nat. Immunol. 2, 1054–1060 - PubMed
-
- D’Eufemia P., Giardini O., Cantani A., Martino F., Finocchiaro R. (1992). Autoimmune thyroiditis in a case of tyrosinaemia type III. J. Inherit. Metab. Dis. 15, 861–862 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

