Cyclooxygenase-2, not microsomal prostaglandin E synthase-1, is the mechanism for interleukin-1β-induced prostaglandin E2 production and inhibition of insulin secretion in pancreatic islets
- PMID: 22822059
- PMCID: PMC3442555
- DOI: 10.1074/jbc.M112.364612
Cyclooxygenase-2, not microsomal prostaglandin E synthase-1, is the mechanism for interleukin-1β-induced prostaglandin E2 production and inhibition of insulin secretion in pancreatic islets
Abstract
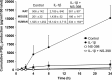
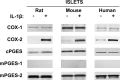
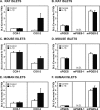
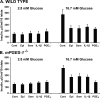
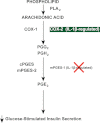
Arachidonic acid is converted to prostaglandin E(2) (PGE(2)) by a sequential enzymatic reaction performed by two isoenzyme groups, cyclooxygenases (COX-1 and COX-2) and terminal prostaglandin E synthases (cPGES, mPGES-1, and mPGES-2). mPGES-1 is widely considered to be the final enzyme regulating COX-2-dependent PGE(2) synthesis. These generalizations have been based in most part on experiments utilizing gene expression analyses of cell lines and tumor tissue. To assess the relevance of these generalizations to a native mammalian tissue, we used isolated human and rodent pancreatic islets to examine interleukin (IL)-1β-induced PGE(2) production, because PGE(2) has been shown to mediate IL-1β inhibition of islet function. Rat islets constitutively expressed mRNAs of COX-1, COX-2, cPGES, and mPGES-1. As expected, IL-1β increased mRNA levels for COX-2 and mPGES-1, but not for COX-1 or cPGES. Basal protein levels of COX-1, cPGES, and mPGES-2 were readily detected in whole cell extracts but were not regulated by IL-1β. IL-1β increased protein levels of COX-2, but unexpectedly mPGES-1 protein levels were low and unaffected. In microsomal extracts, mPGES-1 protein was barely detectable in rat islets but clearly present in human islets; however, in neither case did IL-1β increase mPGES-1 protein levels. To further assess the importance of mPGES-1 to IL-1β regulation of an islet physiologic response, glucose-stimulated insulin secretion was examined in isolated islets of WT and mPGES-1-deficient mice. IL-1β inhibited glucose-stimulated insulin secretion equally in both WT and mPGES-1(-/-) islets, indicating that COX-2, not mPGES-1, mediates IL-1β-induced PGE(2) production and subsequent inhibition of insulin secretion.
Figures


Similar articles
-
Expression of prostaglandin E synthases in periodontitis immunolocalization and cellular regulation.Am J Pathol. 2011 Apr;178(4):1676-88. doi: 10.1016/j.ajpath.2010.12.048. Am J Pathol. 2011. PMID: 21435451 Free PMC article.
-
Tumor cells induce COX-2 and mPGES-1 expression in microvascular endothelial cells mainly by means of IL-1 receptor activation.Microvasc Res. 2011 May;81(3):261-8. doi: 10.1016/j.mvr.2011.01.006. Epub 2011 Jan 26. Microvasc Res. 2011. PMID: 21277871
-
Microsomal prostaglandin E synthase-1 deficiency is associated with elevated peroxisome proliferator-activated receptor gamma: regulation by prostaglandin E2 via the phosphatidylinositol 3-kinase and Akt pathway.J Biol Chem. 2007 Feb 23;282(8):5356-66. doi: 10.1074/jbc.M610153200. Epub 2006 Dec 22. J Biol Chem. 2007. PMID: 17186945
-
Membrane prostaglandin E synthase-1: a novel therapeutic target.Pharmacol Rev. 2007 Sep;59(3):207-24. doi: 10.1124/pr.59.3.1. Pharmacol Rev. 2007. PMID: 17878511 Review.
-
Identification and development of mPGES-1 inhibitors: where we are at?Future Med Chem. 2011 Nov;3(15):1909-34. doi: 10.4155/fmc.11.136. Future Med Chem. 2011. PMID: 22023034 Free PMC article. Review.
Cited by
-
Diabetes Associated Metabolomic Perturbations in NOD Mice.Metabolomics. 2015 Apr;11(2):425-437. doi: 10.1007/s11306-014-0706-2. Metabolomics. 2015. PMID: 25755629 Free PMC article.
-
Regulation of iNOS gene transcription by IL-1β and IFN-γ requires a coactivator exchange mechanism.Mol Endocrinol. 2013 Oct;27(10):1724-42. doi: 10.1210/me.2013-1159. Epub 2013 Sep 6. Mol Endocrinol. 2013. PMID: 24014650 Free PMC article.
-
Potential roles of GPR120 and its agonists in the management of diabetes.Drug Des Devel Ther. 2014 Jul 29;8:1013-27. doi: 10.2147/DDDT.S53892. eCollection 2014. Drug Des Devel Ther. 2014. PMID: 25114508 Free PMC article. Review.
-
Systemic alterations in the metabolome of diabetic NOD mice delineate increased oxidative stress accompanied by reduced inflammation and hypertriglyceremia.Am J Physiol Endocrinol Metab. 2015 Jun 1;308(11):E978-89. doi: 10.1152/ajpendo.00019.2015. Epub 2015 Apr 7. Am J Physiol Endocrinol Metab. 2015. PMID: 25852003 Free PMC article.
-
Human Islet Expression Levels of Prostaglandin E2 Synthetic Enzymes, But Not Prostaglandin EP3 Receptor, Are Positively Correlated with Markers of β-Cell Function and Mass in Nondiabetic Obesity.ACS Pharmacol Transl Sci. 2021 Jun 16;4(4):1338-1348. doi: 10.1021/acsptsci.1c00045. eCollection 2021 Aug 13. ACS Pharmacol Transl Sci. 2021. PMID: 34423270 Free PMC article.
References
-
- Rosen G. D., Birkenmeier T. M., Raz A., Holtzman M. J. (1989) Identification of a cyclooxygenase-related gene and its potential role in prostaglandin formation. Biochem. Biophys. Res. Commun. 164, 1358–1365 - PubMed
-
- Fu J. Y., Masferrer J. L., Seibert K., Raz A., Needleman P. (1990) The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J. Biol. Chem. 265, 16737–16740 - PubMed
-
- Herschman H. R. (1996) Prostaglandin synthase 2. Biochim. Biophys. Acta 1299, 125–140 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

