Catalytic convergence of manganese and iron lipoxygenases by replacement of a single amino acid
- PMID: 22822060
- PMCID: PMC3442510
- DOI: 10.1074/jbc.M112.364331
Catalytic convergence of manganese and iron lipoxygenases by replacement of a single amino acid
Abstract
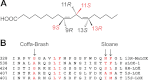
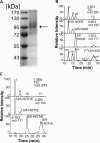
Lipoxygenases (LOXs) contain a hydrophobic substrate channel with the conserved Gly/Ala determinant of regio- and stereospecificity and a conserved Leu residue near the catalytic non-heme iron. Our goal was to study the importance of this region (Gly(332), Leu(336), and Phe(337)) of a lipoxygenase with catalytic manganese (13R-MnLOX). Recombinant 13R-MnLOX oxidizes 18:2n-6 and 18:3n-3 to 13R-, 11(S or R)-, and 9S-hydroperoxy metabolites (∼80-85, 15-20, and 2-3%, respectively) by suprafacial hydrogen abstraction and oxygenation. Replacement of Phe(337) with Ile changed the stereochemistry of the 13-hydroperoxy metabolites of 18:2n-6 and 18:3n-3 (from ∼100% R to 69-74% S) with little effect on regiospecificity. The abstraction of the pro-S hydrogen of 18:2n-6 was retained, suggesting antarafacial hydrogen abstraction and oxygenation. Replacement of Leu(336) with smaller hydrophobic residues (Val, Ala, and Gly) shifted the oxygenation from C-13 toward C-9 with formation of 9S- and 9R-hydroperoxy metabolites of 18:2n-6 and 18:3n-3. Replacement of Gly(332) and Leu(336) with larger hydrophobic residues (G332A and L336F) selectively augmented dehydration of 13R-hydroperoxyoctadeca-9Z,11E,15Z-trienoic acid and increased the oxidation at C-13 of 18:1n-6. We conclude that hydrophobic replacements of Leu(336) can modify the hydroperoxide configurations at C-9 with little effect on the R configuration at C-13 of the 18:2n-6 and 18:3n-3 metabolites. Replacement of Phe(337) with Ile changed the stereospecific oxidation of 18:2n-6 and 18:3n-3 with formation of 13S-hydroperoxides by hydrogen abstraction and oxygenation in analogy with soybean LOX-1.
Figures





Similar articles
-
A G316A mutation of manganese lipoxygenase augments hydroperoxide isomerase activity: mechanism of biosynthesis of epoxyalcohols.J Biol Chem. 2006 Jun 30;281(26):17612-23. doi: 10.1074/jbc.M510311200. Epub 2006 Apr 26. J Biol Chem. 2006. PMID: 16641090
-
Kinetic investigation of the rate-limiting step of manganese- and iron-lipoxygenases.Arch Biochem Biophys. 2014 Aug;555-556:9-15. doi: 10.1016/j.abb.2014.05.014. Epub 2014 May 21. Arch Biochem Biophys. 2014. PMID: 24857825
-
A single active site residue directs oxygenation stereospecificity in lipoxygenases: stereocontrol is linked to the position of oxygenation.Proc Natl Acad Sci U S A. 2004 Nov 2;101(44):15579-84. doi: 10.1073/pnas.0406727101. Epub 2004 Oct 20. Proc Natl Acad Sci U S A. 2004. PMID: 15496467 Free PMC article.
-
Iron and manganese lipoxygenases of plant pathogenic fungi and their role in biosynthesis of jasmonates.Arch Biochem Biophys. 2022 Jun 15;722:109169. doi: 10.1016/j.abb.2022.109169. Epub 2022 Mar 8. Arch Biochem Biophys. 2022. PMID: 35276213 Review.
-
Plant and fungal lipoxygenases.Prostaglandins Other Lipid Mediat. 2002 Aug;68-69:313-23. doi: 10.1016/s0090-6980(02)00037-0. Prostaglandins Other Lipid Mediat. 2002. PMID: 12432926 Review.
Cited by
-
Crystallization and preliminary crystallographic analysis of manganese lipoxygenase.Acta Crystallogr F Struct Biol Commun. 2014 Apr;70(Pt 4):522-5. doi: 10.1107/S2053230X14005548. Epub 2014 Mar 25. Acta Crystallogr F Struct Biol Commun. 2014. PMID: 24699754 Free PMC article.
-
An iron 13S-lipoxygenase with an α-linolenic acid specific hydroperoxidase activity from Fusarium oxysporum.PLoS One. 2013 May 31;8(5):e64919. doi: 10.1371/journal.pone.0064919. Print 2013. PLoS One. 2013. PMID: 23741422 Free PMC article.
-
EPR Spectroscopic Studies of Lipoxygenases.Chem Asian J. 2020 Jan 2;15(1):42-50. doi: 10.1002/asia.201901461. Epub 2019 Dec 5. Chem Asian J. 2020. PMID: 31782616 Free PMC article. Review.
-
Manganese lipoxygenase of F. oxysporum and the structural basis for biosynthesis of distinct 11-hydroperoxy stereoisomers.J Lipid Res. 2015 Aug;56(8):1606-15. doi: 10.1194/jlr.M060178. Epub 2015 Jun 25. J Lipid Res. 2015. PMID: 26113537 Free PMC article.
-
Crystal structure of linoleate 13R-manganese lipoxygenase in complex with an adhesion protein.J Lipid Res. 2016 Aug;57(8):1574-88. doi: 10.1194/jlr.M069617. Epub 2016 Jun 15. J Lipid Res. 2016. PMID: 27313058 Free PMC article.
References
-
- Andreou A., Feussner I. (2009) Lipoxygenases—structure and reaction mechanism. Phytochemistry 70, 1504–1510 - PubMed
-
- Ivanov I., Heydeck D., Hofheinz K., Roffeis J., O'Donnell V. B., Kuhn H., Walther M. (2010) Molecular enzymology of lipoxygenases. Arch. Biochem. Biophys. 503, 161–174 - PubMed
-
- Haeggström J. Z., Funk C. D. (2011) Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem. Rev. 111, 5866–5898 - PubMed
-
- Brodhun F., Feussner I. (2011) Oxylipins in fungi. FEBS J. 278, 1047–1063 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

