Leucine-rich repeat 11 of Toll-like receptor 9 can tightly bind to CpG-containing oligodeoxynucleotides, and the positively charged residues are critical for the high affinity
- PMID: 22822061
- PMCID: PMC3436306
- DOI: 10.1074/jbc.M112.396432
Leucine-rich repeat 11 of Toll-like receptor 9 can tightly bind to CpG-containing oligodeoxynucleotides, and the positively charged residues are critical for the high affinity
Abstract
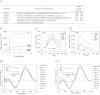
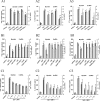
TLR9 is a receptor for sensing bacterial DNA/CpG-containing oligonucleotides (CpG ODN). The extracellular domain (ECD) of human TLR9 (hTLR9) is composed of 25 leucine-rich repeats (LRR) contributing to the binding of CpG ODN. Herein, we showed that among LRR2, -5, -8, and -11, LRR11 of hTLR9 had the highest affinity for CpG ODN followed by LRR2 and -5, whereas LRR8 had almost no affinity. In vitro, preincubation with LRR11 more significantly decreased CpG ODN internalization, subsequent NF-κB activation, and cytokine release than with LRR2 and -5 in mouse peritoneal macrophages treated with CpG ODN. The LRR11 deletion mutant of hTLR9 conferred decreased cellular responses to CpG ODN. Single- or multiple-site mutants at five positively charged residues of LRR11 (LRR11m1-9), especially Arg-337 and Lys-367, were shown to contribute to hTLR9 binding of CpG ODN. LRR11m1-9 showed reduced inhibition of CpG ODN internalization and CpG ODN/TLR9 signaling, supporting the above findings. Prediction of whole hTLR9 ECD-CpG ODN interactions revealed that Arg-337 and Lys-338 directly contact CpG ODN through hydrogen bonding, whereas Lys-347, Arg-348, and His-353 contribute to stabilizing the shape of the ligand binding region. These findings suggested that although all five positively charged residues within LRR11 contributed to its high affinity, only Arg-337 and Lys-338 directly interacted with CpG ODN. In conclusion, the results suggested that LRR11 could strongly bind to CpG ODN, whereas mutations at the five positively charge residues reduced this high affinity. LRR11 may be further investigated as an antagonist of hTLR9.
Figures








Similar articles
-
Synthetic Human TLR9-LRR11 Peptide Attenuates TLR9 Signaling by Binding to and thus Decreasing Internalization of CpG Oligodeoxynucleotides.Int J Mol Sci. 2016 Feb 22;17(2):242. doi: 10.3390/ijms17020242. Int J Mol Sci. 2016. PMID: 26907260 Free PMC article.
-
Toll-like receptor 9 interaction with CpG ODN--an in silico analysis approach.Theor Biol Med Model. 2013 Mar 14;10:18. doi: 10.1186/1742-4682-10-18. Theor Biol Med Model. 2013. PMID: 23497207 Free PMC article.
-
Recognition of CpG oligodeoxynucleotides by human Toll-like receptor 9 and subsequent cytokine induction.Biochem Biophys Res Commun. 2013 Jan 25;430(4):1234-9. doi: 10.1016/j.bbrc.2012.12.068. Epub 2012 Dec 22. Biochem Biophys Res Commun. 2013. PMID: 23266611
-
DNA motifs suppressing TLR9 responses.Crit Rev Immunol. 2006;26(6):527-44. doi: 10.1615/critrevimmunol.v26.i6.50. Crit Rev Immunol. 2006. PMID: 17341193 Review.
-
Immunostimulatory Activities of CpG-Oligodeoxynucleotides in Teleosts: Toll-Like Receptors 9 and 21.Front Immunol. 2019 Feb 8;10:179. doi: 10.3389/fimmu.2019.00179. eCollection 2019. Front Immunol. 2019. PMID: 30800129 Free PMC article. Review.
Cited by
-
Development of CpG-oligodeoxynucleotides for effective activation of rabbit TLR9 mediated immune responses.PLoS One. 2014 Sep 30;9(9):e108808. doi: 10.1371/journal.pone.0108808. eCollection 2014. PLoS One. 2014. PMID: 25269083 Free PMC article.
-
Magic Peptide: Unique Properties of the LRR11 Peptide in the Activation of Leukotriene Synthesis in Human Neutrophils.Int J Mol Sci. 2021 Mar 6;22(5):2671. doi: 10.3390/ijms22052671. Int J Mol Sci. 2021. PMID: 33800897 Free PMC article.
-
CpG Oligodeoxynucleotides Modulate Innate and Adaptive Functions of IgM+ B Cells in Rainbow Trout.Front Immunol. 2019 Mar 26;10:584. doi: 10.3389/fimmu.2019.00584. eCollection 2019. Front Immunol. 2019. PMID: 30972075 Free PMC article.
-
Synthetic Human TLR9-LRR11 Peptide Attenuates TLR9 Signaling by Binding to and thus Decreasing Internalization of CpG Oligodeoxynucleotides.Int J Mol Sci. 2016 Feb 22;17(2):242. doi: 10.3390/ijms17020242. Int J Mol Sci. 2016. PMID: 26907260 Free PMC article.
-
Toll-like receptor 9 interaction with CpG ODN--an in silico analysis approach.Theor Biol Med Model. 2013 Mar 14;10:18. doi: 10.1186/1742-4682-10-18. Theor Biol Med Model. 2013. PMID: 23497207 Free PMC article.
References
-
- Akira S., Uematsu S., Takeuchi O. (2006) Pathogen recognition and innate immunity. Cell 124, 783–801 - PubMed
-
- Uematsu S., Akira S. (2007) Toll-like receptors and Type I interferons. J. Biol. Chem. 282, 15319–15323 - PubMed
-
- Jin M. S., Lee J. O. (2008) Structures of TLR-ligand complexes. Curr. Opin Immunol. 20, 414–419 - PubMed
-
- Akira S., Takeda K. (2004) Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 - PubMed
-
- Bell J. K., Mullen G. E., Leifer C. A., Mazzoni A., Davies D. R., Segal D. M. (2003) Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol. 24, 528–533 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

