Ryanodine receptors: structure and function
- PMID: 22822064
- PMCID: PMC3442496
- DOI: 10.1074/jbc.R112.349068
Ryanodine receptors: structure and function
Abstract
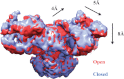
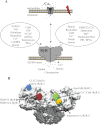
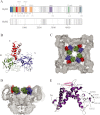
Ryanodine receptors (RyRs) are huge ion channels that are responsible for the release of Ca(2+) from the sarco/endoplasmic reticulum. RyRs form homotetramers with a mushroom-like shape, consisting of a large cytoplasmic head and transmembrane stalk. Ca(2+) is a major physiological ligand that triggers opening of RyRs, but a plethora of modulatory proteins and small molecules in the cytoplasm and sarco/endoplasmic reticulum lumen have been recognized. Over 300 mutations in RyRs are associated with severe skeletal muscle disorders or triggered cardiac arrhythmias. With the advent of high-resolution structures of individual domains, many of these can be mapped onto the three-dimensional structure.
Figures

References
-
- Rogers E. F., Koniuszy F. R., Shavel J., Jr., Folkersand K. (1948) Plant insecticides. I. Ryanodine, a new alkaloid from Ryania speciosa Vahl. J. Am. Chem. Soc. 70, 3086–3088 - PubMed
-
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. (1988) Purification and reconstitution of the calcium release channel from skeletal muscle. Nature 331, 315–319 - PubMed
-
- Inui M., Saito A., Fleischer S. (1987) Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J. Biol. Chem. 262, 1740–1747 - PubMed
-
- Meissner G. (1986) Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J. Biol. Chem. 261, 6300–6306 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

