Cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) as messengers for calcium mobilization
- PMID: 22822066
- PMCID: PMC3442497
- DOI: 10.1074/jbc.R112.349464
Cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) as messengers for calcium mobilization
Abstract
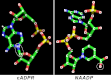
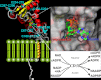
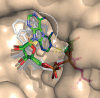
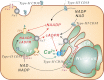
Cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate were discovered >2 decades ago. That they are second messengers for mobilizing Ca(2+) stores has since been firmly established. Separate stores and distinct Ca(2+) channels are targeted, with cyclic ADP-ribose acting on the ryanodine receptors in the endoplasmic reticulum, whereas nicotinic acid adenine dinucleotide phosphate mobilizes the endolysosomes via the two-pore channels. Despite the structural and functional differences, both messengers are synthesized by a ubiquitous enzyme, CD38, whose crystal structure and catalytic mechanism have now been well elucidated. How this novel signaling enzyme is regulated remains largely unknown and is the focus of this minireview.
Figures
Similar articles
-
Resolving the topological enigma in Ca2+ signaling by cyclic ADP-ribose and NAADP.J Biol Chem. 2019 Dec 27;294(52):19831-19843. doi: 10.1074/jbc.REV119.009635. Epub 2019 Oct 31. J Biol Chem. 2019. PMID: 31672920 Free PMC article. Review.
-
Cyclic ADP-ribose and NAADP: fraternal twin messengers for calcium signaling.Sci China Life Sci. 2011 Aug;54(8):699-711. doi: 10.1007/s11427-011-4197-3. Epub 2011 Jul 24. Sci China Life Sci. 2011. PMID: 21786193 Review.
-
Synthesis of the Ca2+-mobilizing messengers NAADP and cADPR by intracellular CD38 enzyme in the mouse heart: Role in β-adrenoceptor signaling.J Biol Chem. 2017 Aug 11;292(32):13243-13257. doi: 10.1074/jbc.M117.789347. Epub 2017 May 24. J Biol Chem. 2017. PMID: 28539361 Free PMC article.
-
A unified mechanism of enzymatic synthesis of two calcium messengers: cyclic ADP-ribose and NAADP.Biol Chem. 1999 Jul-Aug;380(7-8):785-93. doi: 10.1515/BC.1999.098. Biol Chem. 1999. PMID: 10494827 Review.
-
Generation of cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate by CD38 for Ca2+ signaling in interleukin-8-treated lymphokine-activated killer cells.J Biol Chem. 2010 Jul 9;285(28):21877-87. doi: 10.1074/jbc.M109.066290. Epub 2010 May 4. J Biol Chem. 2010. PMID: 20442403 Free PMC article.
Cited by
-
CD38 in the age of COVID-19: a medical perspective.Physiol Rev. 2021 Oct 1;101(4):1457-1486. doi: 10.1152/physrev.00046.2020. Epub 2021 Mar 31. Physiol Rev. 2021. PMID: 33787351 Free PMC article. Review.
-
Resolving the topological enigma in Ca2+ signaling by cyclic ADP-ribose and NAADP.J Biol Chem. 2019 Dec 27;294(52):19831-19843. doi: 10.1074/jbc.REV119.009635. Epub 2019 Oct 31. J Biol Chem. 2019. PMID: 31672920 Free PMC article. Review.
-
The chemistry of the vitamin B3 metabolome.Biochem Soc Trans. 2019 Feb 28;47(1):131-147. doi: 10.1042/BST20180420. Epub 2018 Dec 17. Biochem Soc Trans. 2019. PMID: 30559273 Free PMC article. Review.
-
CD38 produces nicotinic acid adenosine dinucleotide phosphate in the lysosome.J Biol Chem. 2018 May 25;293(21):8151-8160. doi: 10.1074/jbc.RA118.002113. Epub 2018 Apr 9. J Biol Chem. 2018. PMID: 29632067 Free PMC article.
-
Specific cyclic ADP-ribose phosphohydrolase obtained by mutagenic engineering of Mn2+-dependent ADP-ribose/CDP-alcohol diphosphatase.Sci Rep. 2018 Jan 18;8(1):1036. doi: 10.1038/s41598-017-18393-9. Sci Rep. 2018. PMID: 29348648 Free PMC article.
References
-
- Streb H., Irvine R. F., Berridge M. J., Schulz I. (1983) Release of Ca2+ from a non-mitochondrial intracellular store in pancreatic acinar cells by inositol 1,4,5-trisphosphate. Nature 306, 67–69 - PubMed
-
- Lee H. C., Aarhus R. (1995) A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J. Biol. Chem. 270, 2152–2157 - PubMed
-
- Lee H. C., Aarhus R., Levitt D. (1994) The crystal structure of cyclic ADP-ribose. Nat. Struct. Biol. 1, 143–144 - PubMed
-
- Lee H. C., Walseth T. F., Bratt G. T., Hayes R. N., Clapper D. L. (1989) Structural determination of a cyclic metabolite of NAD+ with intracellular Ca2+-mobilizing activity. J. Biol. Chem. 264, 1608–1615 - PubMed
-
- Wu Y., Kuzma J., Maréchal E., Graeff R., Lee H. C., Foster R., Chua N. H. (1997) Abscisic acid signaling through cyclic ADP-ribose in plants. Science 278, 2126–2130 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

