Redefinition of the carbohydrate binding specificity of Helicobacter pylori BabA adhesin
- PMID: 22822069
- PMCID: PMC3442506
- DOI: 10.1074/jbc.M112.387654
Redefinition of the carbohydrate binding specificity of Helicobacter pylori BabA adhesin
Abstract
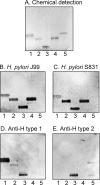
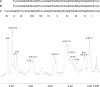
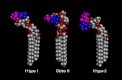
Certain Helicobacter pylori strains adhere to the human gastric epithelium using the blood group antigen-binding adhesin (BabA). All BabA-expressing H. pylori strains bind to the blood group O determinants on type 1 core chains, i.e. to the Lewis b antigen (Fucα2Galβ3(Fucα4)GlcNAc; Le(b)) and the H type 1 determinant (Fucα2Galβ3GlcNAc). Recently, BabA strains have been categorized into those recognizing only Le(b) and H type 1 determinants (designated specialist strains) and those that also bind to A and B type 1 determinants (designated generalist strains). Here, the structural requirements for carbohydrate recognition by generalist and specialist BabA were further explored by binding of these types of strains to a panel of different glycosphingolipids. Three glycosphingolipids recognized by both specialist and generalist BabA were isolated from the small intestine of a blood group O pig and characterized by mass spectrometry and proton NMR as H type 1 pentaglycosylceramide (Fucα2Galβ3GlcNAcβ3Galβ4Glcβ1Cer), Globo H hexaglycosylceramide (Fucα2Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer), and a mixture of three complex glycosphingolipids (Fucα2Galβ4GlcNAcβ6(Fucα2Galβ3GlcNAcβ3)Galβ3GlcNAcβ3Galβ4Glcβ1Cer, Fucα2Galβ3GlcNAcβ6(Fucα2Galβ3GlcNAcβ3)Galβ3GlcNAcβ3Galβ4Glcβ1Cer, and Fucα2Galβ4(Fucα3)GlcNAcβ6(Fucα2Galβ3GlcNAcβ3)Galβ3GlcNAcβ3Galβ4Glcβ1Cer). In addition to the binding of both strains to the Globo H hexaglycosylceramide, i.e. a blood group O determinant on a type 4 core chain, the generalist strain bound to the Globo A heptaglycosylceramide (GalNAcα3(Fucα2)Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer), i.e. a blood group A determinant on a type 4 core chain. The binding of BabA to the two sets of isoreceptors is due to conformational similarities of the terminal disaccharides of H type 1 and Globo H and of the terminal trisaccharides of A type 1 and Globo A.
Figures







References
-
- Karlsson K. A. (1989) Animal glycosphingolipids as membrane attachment sites for bacteria. Annu. Rev. Biochem. 58, 309–350 - PubMed
-
- Esko J. D. (1999) Essentials in Glycobiology (Varki A., Cummings R., Esko J., Freeze H., Hart G., Marth J., eds) pp. 429–440, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY - PubMed
-
- Pieters R. J. (2011) Carbohydrate mediated bacterial adhesion. Adv. Exp. Med. Biol. 715, 227–240 - PubMed
-
- Stults C. L., Sweeley C. C., Macher B. A. (1989) Glycosphingolipids: structure, biological source and properties. Methods Enzymol. 179, 167–214 - PubMed
-
- Borén T., Falk P., Roth K. A., Larson G., Normark S. (1993) Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 262, 1892–1895 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

