Identifying key juxtamembrane interactions in cell membranes using AraC-based transcriptional reporter assay (AraTM)
- PMID: 22822084
- PMCID: PMC3438984
- DOI: 10.1074/jbc.M112.396895
Identifying key juxtamembrane interactions in cell membranes using AraC-based transcriptional reporter assay (AraTM)
Abstract
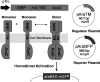
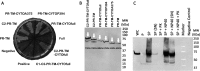
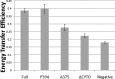
Dimerization is a key regulatory mechanism in activation of transmembrane (TM) receptors during signal transduction. This process involves a coordinated interplay between extracellular (EX), TM, and cytoplasmic (CYTO) regions to form a specific interface required for both ligand binding and intracellular signaling to occur. While several transcriptional activator-based methods exist for investigating TM interactions in bacterial membranes, expression of TM chimera in these methods occurs in a reverse orientation, and are limited to only TM domains for proper membrane trafficking and integration. We therefore developed a new, AraC-based transcriptional reporter assay (AraTM) that expresses EX-TM-CYTO chimera in their native orientation, thereby enabling membrane trafficking to occur independent of the TM chimera used as well as permitting analysis of EX-TM-CYTO interactions in biological membranes. Using integrin α(IIb) TM-CYTO as a model, we observe a large increase in homodimerization for the constitutively active TM mutant L980A relative to wild-type in the TM-CYTO construct (A963-E1008). We also characterized the receptor for advanced glycation endproducts (RAGE), whose homooligomeric state is critical in ligand recognition, and find the specific juxtamembrane region within the CYTO (A375-P394) mediates homodimerization, and is dominant over effects observed when the extracellular C2 domain is included. Furthermore, we find good agreement between our AraTM measurements in bacterial membranes and BRET measurements made on corresponding RAGE constructs expressed in transfected HEK293 cells. Overall, the AraTM assay provides a new approach to identify specific interactions between receptor EX-TM-CYTO domains in biological membranes that are important in regulation of signal transduction.
Figures







Similar articles
-
A novel assay for assessing juxtamembrane and transmembrane domain interactions important for receptor heterodimerization.J Mol Biol. 2013 Nov 15;425(22):4652-8. doi: 10.1016/j.jmb.2013.07.022. Epub 2013 Jul 20. J Mol Biol. 2013. PMID: 23876708
-
A specific, transmembrane interface regulates fibroblast activation protein (FAP) homodimerization, trafficking and exopeptidase activity.Biochim Biophys Acta. 2016 Aug;1858(8):1876-82. doi: 10.1016/j.bbamem.2016.05.001. Epub 2016 May 4. Biochim Biophys Acta. 2016. PMID: 27155568
-
A cytosolic juxtamembrane interface modulates plexin A3 oligomerization and signal transduction.PLoS One. 2015 Jan 7;10(1):e0116368. doi: 10.1371/journal.pone.0116368. eCollection 2015. PLoS One. 2015. PMID: 25565389 Free PMC article.
-
The single transmembrane domains of ErbB receptors self-associate in cell membranes.J Biol Chem. 2002 Feb 15;277(7):4704-12. doi: 10.1074/jbc.M108681200. Epub 2001 Dec 10. J Biol Chem. 2002. PMID: 11741943
-
AraC protein, regulation of the l-arabinose operon in Escherichia coli, and the light switch mechanism of AraC action.FEMS Microbiol Rev. 2010 Sep;34(5):779-96. doi: 10.1111/j.1574-6976.2010.00226.x. Epub 2010 Apr 8. FEMS Microbiol Rev. 2010. PMID: 20491933 Review.
Cited by
-
Promoting the activity of a receptor tyrosine phosphatase with a novel pH-responsive transmembrane agonist inhibits cancer-associated phenotypes.Protein Sci. 2023 Sep;32(9):e4742. doi: 10.1002/pro.4742. Protein Sci. 2023. PMID: 37515426 Free PMC article.
-
Membrane-enabled dimerization of the intrinsically disordered cytoplasmic domain of ADAM10.Proc Natl Acad Sci U S A. 2014 Nov 11;111(45):15987-92. doi: 10.1073/pnas.1409354111. Epub 2014 Oct 27. Proc Natl Acad Sci U S A. 2014. PMID: 25349418 Free PMC article.
-
Identifying Transmembrane Interactions in Receptor Protein Tyrosine Phosphatase Homodimerization and Heterodimerization.Methods Mol Biol. 2024;2743:195-209. doi: 10.1007/978-1-0716-3569-8_13. Methods Mol Biol. 2024. PMID: 38147217 Free PMC article.
-
Applications of Single-Molecule Methods to Membrane Protein Folding Studies.J Mol Biol. 2018 Feb 16;430(4):424-437. doi: 10.1016/j.jmb.2017.05.021. Epub 2017 May 23. J Mol Biol. 2018. PMID: 28549924 Free PMC article. Review.
-
Disrupting PTPRJ transmembrane-mediated oligomerization counteracts oncogenic receptor tyrosine kinase FLT3 ITD.Front Oncol. 2022 Nov 14;12:1017947. doi: 10.3389/fonc.2022.1017947. eCollection 2022. Front Oncol. 2022. PMID: 36452504 Free PMC article.
References
-
- Klemm J. D., Schreiber S. L., Crabtree G. R. (1998) Dimerization as a regulatory mechanism in signal transduction. Annu. Rev. Immunol. 16, 569–592 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

