Protein carbonylation and adipocyte mitochondrial function
- PMID: 22822087
- PMCID: PMC3463318
- DOI: 10.1074/jbc.M112.400663
Protein carbonylation and adipocyte mitochondrial function
Abstract
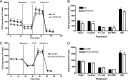
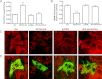
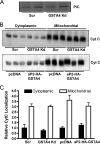
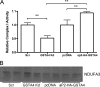
Carbonylation is the covalent, non-reversible modification of the side chains of cysteine, histidine, and lysine residues by lipid peroxidation end products such as 4-hydroxy- and 4-oxononenal. In adipose tissue the effects of such modifications are associated with increased oxidative stress and metabolic dysregulation centered on mitochondrial energy metabolism. To address the role of protein carbonylation in the pathogenesis of mitochondrial dysfunction, quantitative proteomics was employed to identify specific targets of carbonylation in GSTA4-silenced or overexpressing 3T3-L1 adipocytes. GSTA4-silenced adipocytes displayed elevated carbonylation of several key mitochondrial proteins including the phosphate carrier protein, NADH dehydrogenase 1α subcomplexes 2 and 3, translocase of inner mitochondrial membrane 50, and valyl-tRNA synthetase. Elevated protein carbonylation is accompanied by diminished complex I activity, impaired respiration, increased superoxide production, and a reduction in membrane potential without changes in mitochondrial number, area, or density. Silencing of the phosphate carrier or NADH dehydrogenase 1α subcomplexes 2 or 3 in 3T3-L1 cells results in decreased basal and maximal respiration. These results suggest that protein carbonylation plays a major instigating role in cytokine-dependent mitochondrial dysfunction and may be linked to the development of insulin resistance in the adipocyte.
Figures




References
-
- Bonadonna R. C., Groop L., Kraemer N., Ferrannini E., Del Prato S., DeFronzo R. A. (1990) Obesity and insulin resistance in humans. A dose-response study. Metab. Clin. Exp. 39, 452–459 - PubMed
-
- Houstis N., Rosen E. D., Lander E. (2006) Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440, 944–948 - PubMed
-
- Fang J., Holmgren A. (2006) Inhibition of thioredoxin and thioredoxin reductase by 4-hydroxy-2-nonenal in vitro and in vivo. J. Am. Chem. Soc. 128, 1879–1885 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

