Neuregulin and dopamine modulation of hippocampal gamma oscillations is dependent on dopamine D4 receptors
- PMID: 22822214
- PMCID: PMC3420189
- DOI: 10.1073/pnas.1201011109
Neuregulin and dopamine modulation of hippocampal gamma oscillations is dependent on dopamine D4 receptors
Abstract
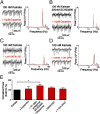
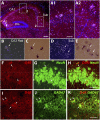
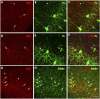
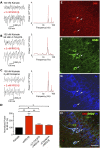
The neuregulin/ErbB signaling network is genetically associated with schizophrenia and modulates hippocampal γ oscillations--a type of neuronal network activity important for higher brain processes and altered in psychiatric disorders. Because neuregulin-1 (NRG-1) dramatically increases extracellular dopamine levels in the hippocampus, we investigated the relationship between NRG/ErbB and dopamine signaling in hippocampal γ oscillations. Using agonists for different D1- and D2-type dopamine receptors, we found that the D4 receptor (D4R) agonist PD168077, but not D1/D5 and D2/D3 agonists, increases γ oscillation power, and its effect is blocked by the highly specific D4R antagonist L-745,870. Using double in situ hybridization and immunofluorescence histochemistry, we show that hippocampal D4R mRNA and protein are more highly expressed in GAD67-positive GABAergic interneurons, many of which express the NRG-1 receptor ErbB4. Importantly, D4 and ErbB4 receptors are coexpressed in parvalbumin-positive basket cells that are critical for γ oscillations. Last, we report that D4R activation is essential for the effects of NRG-1 on network activity because L-745,870 and the atypical antipsychotic clozapine dramatically reduce the NRG-1-induced increase in γ oscillation power. This unique link between D4R and ErbB4 signaling on γ oscillation power, and their coexpression in parvalbumin-expressing interneurons, suggests a cellular mechanism that may be compromised in different psychiatric disorders affecting cognitive control. These findings are important given the association of a DRD4 polymorphism with alterations in attention, working memory, and γ oscillations, and suggest potential benefits of D4R modulators for targeting cognitive deficits.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. - PubMed
-
- Colgin LL, Moser EI. Gamma oscillations in the hippocampus. Physiology (Bethesda) 2010;25:319–329. - PubMed
-
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

