Heat shock protein 90 is required for conidiation and cell wall integrity in Aspergillus fumigatus
- PMID: 22822234
- PMCID: PMC3486032
- DOI: 10.1128/EC.00032-12
Heat shock protein 90 is required for conidiation and cell wall integrity in Aspergillus fumigatus
Abstract
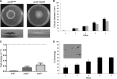
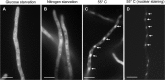
Heat shock protein 90 (Hsp90) is a eukaryotic molecular chaperone. Its involvement in the resistance of Candida albicans to azole and echinocandin antifungals is well established. However, little is known about Hsp90's function in the filamentous fungal pathogen Aspergillus fumigatus. We investigated the role of Hsp90 in A. fumigatus by genetic repression and examined its cellular localization under various stress conditions. Failure to generate a deletion strain of hsp90 suggested that it is essential. Genetic repression of Hsp90 was achieved by an inducible nitrogen-dependent promoter (pniiA-Hsp90) and led to decreased spore viability, decreased hyphal growth, and severe defects in germination and conidiation concomitant with the downregulation of the conidiation-specific transcription factors brlA, wetA, and abaA. Hsp90 repression potentiated the effect of cell wall inhibitors affecting the β-glucan structure of the cell wall (caspofungin, Congo red) and of the calcineurin inhibitor FK506, supporting a role in regulating cell wall integrity pathways. Moreover, compromising Hsp90 abolished the paradoxical effect of caspofungin. Pharmacological inhibition of Hsp90 by geldanamycin and its derivatives (17-AAG and 17-DMAG) resulted in similar effects. C-terminal green fluorescent protein (GFP) tagging of Hsp90 revealed mainly cytosolic distribution under standard growth conditions. However, treatment with caspofungin resulted in Hsp90 accumulation at the cell wall and at sites of septum formation, further highlighting its role in cell wall stress compensatory mechanisms. Targeting Hsp90 with fungal-specific inhibitors to cripple stress response compensatory pathways represents an attractive new antifungal strategy.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

