Relationship between side-chain branching and stoichiometry in β(3)-peptide bundles
- PMID: 22822272
- PMCID: PMC3398705
- DOI: 10.1016/j.tet.2012.03.079
Relationship between side-chain branching and stoichiometry in β(3)-peptide bundles
Abstract
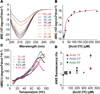
The stability and stoichiometry of β(3)-peptide bundles is influenced by side-chain identity. β(3)-peptides containing β(3)-homoleucine on one helical face assemble into octamers, whereas those containing β(3)-homovaline form tetramers. From a structural perspective, the side chains of β(3)-homoleucine and β(3)-homovaline differ in terms of both side-chain length and γ-carbon branching. To evaluate the extent to which these two parameters control β(3)-peptide bundle stoichiometry, we synthesized the β(3)-peptide Acid-3Y, which contains β(3)-homoisoleucine in place of β(3)-homoleucine or β(3)-homovaline. Acid-3Y assembles into a stable tetramer whose stability resembles that of the previously characterized Acid-VY tetramer. These results suggest that β(3)-peptide bundle stoichiometry is dominated by the presence or absence of γ-carbon branching on core side chains.
Figures


References
-
- Craig CJ, Goodman JL, Schepartz A. ChemBioChem. 2011;12:1035–1038. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
