Monoclonal antibodies and toxins--a perspective on function and isotype
- PMID: 22822456
- PMCID: PMC3398419
- DOI: 10.3390/toxins4060430
Monoclonal antibodies and toxins--a perspective on function and isotype
Abstract
Antibody therapy remains the only effective treatment for toxin-mediated diseases. The development of hybridoma technology has allowed the isolation of monoclonal antibodies (mAbs) with high specificity and defined properties, and numerous mAbs have been purified and characterized for their protective efficacy against different toxins. This review summarizes the mAb studies for 6 toxins--Shiga toxin, pertussis toxin, anthrax toxin, ricin toxin, botulinum toxin, and Staphylococcal enterotoxin B (SEB)--and analyzes the prevalence of mAb functions and their isotypes. Here we show that most toxin-binding mAbs resulted from immunization are non-protective and that mAbs with potential therapeutic use are preferably characterized. Various common practices and caveats of protection studies are discussed, with the goal of providing insights for the design of future research on antibody-toxin interactions.
Keywords: animal model; antibody; clearance; disease enhancement; in vivo; isotype; neutralization; protection; therapeutics; vaccine.
Figures
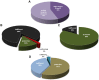
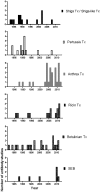
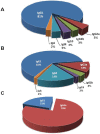
Similar articles
-
Mechanisms mediating enhanced neutralization efficacy of staphylococcal enterotoxin B by combinations of monoclonal antibodies.J Biol Chem. 2015 Mar 13;290(11):6715-30. doi: 10.1074/jbc.M114.630715. Epub 2015 Jan 8. J Biol Chem. 2015. PMID: 25572397 Free PMC article.
-
Immunoglobulin G3 blocking antibodies to the fungal pathogen Cryptococcus neoformans.J Exp Med. 1996 Apr 1;183(4):1905-9. doi: 10.1084/jem.183.4.1905. J Exp Med. 1996. PMID: 8666947 Free PMC article.
-
Prophylaxis and therapy of inhalational anthrax by a novel monoclonal antibody to protective antigen that mimics vaccine-induced immunity.Infect Immun. 2006 Oct;74(10):5840-7. doi: 10.1128/IAI.00712-06. Infect Immun. 2006. PMID: 16988263 Free PMC article.
-
Isotype selection for antibody-based cancer therapy.Clin Exp Immunol. 2021 Mar;203(3):351-365. doi: 10.1111/cei.13545. Epub 2020 Nov 30. Clin Exp Immunol. 2021. PMID: 33155272 Free PMC article. Review.
-
Hybridoma technology a versatile method for isolation of monoclonal antibodies, its applicability across species, limitations, advancement and future perspectives.Int Immunopharmacol. 2020 Aug;85:106639. doi: 10.1016/j.intimp.2020.106639. Epub 2020 May 27. Int Immunopharmacol. 2020. PMID: 32473573 Free PMC article. Review.
Cited by
-
Role of Fc in antibody-mediated protection from ricin toxin.Toxins (Basel). 2014 May 7;6(5):1512-25. doi: 10.3390/toxins6051512. Toxins (Basel). 2014. PMID: 24811206 Free PMC article.
-
Immunization with non-toxic variants of Shiga toxin type 2 (Stx2) generates high titers of protective antibodies.Dokl Biochem Biophys. 2015;460:23-5. doi: 10.1134/S160767291501007X. Epub 2015 Mar 13. Dokl Biochem Biophys. 2015. PMID: 25772984 No abstract available.
-
Oral Passive Immunization With Plasma-Derived Polyreactive Secretory-Like IgA/M Partially Protects Mice Against Experimental Salmonellosis.Front Immunol. 2018 Dec 18;9:2970. doi: 10.3389/fimmu.2018.02970. eCollection 2018. Front Immunol. 2018. PMID: 30619327 Free PMC article.
-
Preparation and evaluation of human-murine chimeric antibody against protective antigen of Bacillus anthracis.Int J Mol Sci. 2014 Oct 14;15(10):18496-507. doi: 10.3390/ijms151018496. Int J Mol Sci. 2014. PMID: 25318053 Free PMC article.
-
The molecular mechanism of Shiga toxin Stx2e neutralization by a single-domain antibody targeting the cell receptor-binding domain.J Biol Chem. 2014 Sep 5;289(36):25374-81. doi: 10.1074/jbc.M114.566257. Epub 2014 Jul 22. J Biol Chem. 2014. PMID: 25053417 Free PMC article.
References
-
- Brock T.D. Milestones in Microbiology: 1556 to 1940. ASM Press; Washington, DC, USA: 1998.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

