T regulatory cells play a significant role in modulating MHC class I antibody-induced obliterative airway disease
- PMID: 22822907
- PMCID: PMC3459183
- DOI: 10.1111/j.1600-6143.2012.04191.x
T regulatory cells play a significant role in modulating MHC class I antibody-induced obliterative airway disease
Abstract
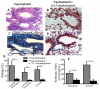
The molecular mechanisms leading to the development of chronic lung allograft dysfunction following de novo development of antibodies to mismatched donor MHC remain undefined. We demonstrated that intrabronchial administration of antibodies to MHC class I resulted in induction of both innate and adaptive cellular immune responses characterized by a predominance of Th17 specific to lung associated self-antigens Kα1-tubulin and Collagen-V leading to the development of obliterative airway lesions (OAD), correlate of chronic rejection following human lung transplantation. To determine the role of regulatory T cells (Treg) in the pathogenesis of OAD, we administered anti-MHC class I to mice, in which Treg were depleted by conditional ablation of FoxP3+cells. Under this condition, we observed a threefold increase in pulmonary cellular infiltration, luminal occlusion and fibrous deposition when compared anti-MHC class I Ab administered mice maintaining FoxP3. OAD lesions were accompanied with enhanced accumulation of neutrophils along with self-antigen-specific Th17 and humoral responses. However, IL-17-blockade or adoptive transfer of Treg abrogated OAD. We conclude that Treg exerts a suppressive effect on anti-MHC induced IL-8-mediated neutrophil infiltration and innate immune responses that leads to inhibition of Th17 immune responses to lung associated self-antigens which is critical for development of OAD.
© Copyright 2012 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures
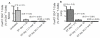
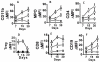

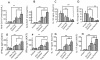
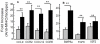


References
-
- Boehler A, Estenne M. Post-transplant bronchiolitis obliterans. Eur Respir J. 2003;22:1007–1018. - PubMed
-
- Boehler A, Kesten S, Weder W, Speich R. Bronchiolitis obliterans after lung transplantation: a review. Chest. 1998;114:1411–1426. - PubMed
-
- Hachem RR, Trulock EP. Bronchiolitis obliterans syndrome: pathogenesis and management. Semin Thorac Cardiovasc Surg. 2004;16:350–355. - PubMed
-
- Trulock EP, Edwards LB, Taylor DO, Boucek MM, Mohacsi PJ, Keck BM, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: Twentieth Official adult lung and heart-lung transplant report--2003. J Heart Lung Transplant. 2003;22:625–635. - PubMed
-
- Smith MA, Sundaresan S, Mohanakumar T, Trulock EP, Lynch JP, Phelan DL, Cooper JD, Patterson GA. Effect of development of antibodies to HLA and cytomegalovirus mismatch on lung transplantation survival and development of bronchiolitis obliterans syndrome. J Thorac Cardiovasc Surg. 1998;116:812–820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

