Maternal effects on male weaponry: female dung beetles produce major sons with longer horns when they perceive higher population density
- PMID: 22823456
- PMCID: PMC3506554
- DOI: 10.1186/1471-2148-12-118
Maternal effects on male weaponry: female dung beetles produce major sons with longer horns when they perceive higher population density
Abstract
Background: Maternal effects are environmental influences on the phenotype of one individual that are due to the expression of genes in its mother, and are expected to evolve whenever females are better capable of assessing the environmental conditions that their offspring will experience than the offspring themselves. In the dung beetle Onthophagus taurus, conditional male dimorphism is associated with alternative reproductive tactics: majors fight and guard females whereas minors sneak copulations. Furthermore, variation in dung beetle population density has different fitness consequences for each male morph, and theory predicts that higher population density might select for a higher frequency of minors and/or greater expenditure on weaponry in majors. Because adult dung beetles provide offspring with all the nutritional resources for their development, maternal effects strongly influence male phenotype.
Results: Here we tested whether female O. taurus are capable of perceiving population density, and responding by changing the phenotype of their offspring. We found that mothers who were reared with other conspecifics in their pre-mating period produced major offspring that had longer horns across a wider range of body sizes than the major offspring of females that were reared in isolation in their pre-mating period. Moreover, our results indicate that this maternal effect on male weaponry does not operate through the amount of dung provided by females to their offspring, but is rather transmitted through egg or brood mass composition. Finally, although theory predicts that females experiencing higher density might produce more minor males, we found no support for this, rather the best fitting models were equivocal as to whether fewer or the same proportions of minors were produced.
Conclusions: Our study describes a new type of maternal effect in dung beetles, which probably allows females to respond to population density adaptively, preparing at least their major offspring for the sexual competition they will face in the future. This new type of maternal effect in dung beetles represents a novel transgenerational response of alternative reproductive tactics to population density.
Figures
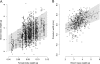


References
-
- Bernardo J. Maternal effects in animal ecology. Am Zool. 1996;36(2):83–105.
-
- Mousseau TA, Dingle H. Maternal effects in insect life histories. Ann Rev Entomol. 1991;36:511–534. doi: 10.1146/annurev.en.36.010191.002455. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

