A new liver perfusion and preservation system for transplantation research in large animals
- PMID: 2282350
- PMCID: PMC2956500
- DOI: 10.3109/08941939009140337
A new liver perfusion and preservation system for transplantation research in large animals
Abstract
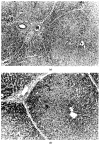
A kidney perfusion machine, model MOX-100 (Waters Instruments, Ltd, Rochester, MN) was modified to allow continuous perfusion of the portal vein and pulsatile perfusion of the hepatic artery of the liver. Additional apparatus consists of a cooling system, a membrane oxygenator, a filter for foreign bodies, and bubble traps. This system not only allows hypothermic perfusion preservation of the liver graft, but furthermore enables investigation of ex vivo simulation of various circulatory circumstances in which physiological perfusion of the liver is studied. We have used this system to evaluate the viability of liver allografts preserved by cold storage. The liver was placed on the perfusion system and perfused with blood with a hematocrit of approximately 20%, and maintained at 37 degrees C for 3 h. The flows of the hepatic artery and portal vein were adjusted to 0.33 mL and 0.67 mL/g of liver tissue, respectively. Parameters of viability consisted of hourly bile output, oxygen consumption, liver enzymes, electrolytes, vascular resistance, and liver histology. This method of liver assessment in large animals will allow the objective evaluation of organ viability for transplantation and thereby improve the outcome of organ transplantation. Furthermore, this pump enables investigation into the pathophysiology of liver ischemia and preservation.
Figures






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
