Incidence and economic burden of adverse drug reactions among elderly patients in Ontario emergency departments: a retrospective study
- PMID: 22823502
- PMCID: PMC3714138
- DOI: 10.1007/BF03261973
Incidence and economic burden of adverse drug reactions among elderly patients in Ontario emergency departments: a retrospective study
Abstract
Background: The rapid rise in the availability and use of pharmaceutical agents, and particularly polypharmacy, directly increases the risk for patients to experience adverse drug reactions (ADRs). There are few studies on the overall incidence and costs of ADRs.
Objective: The aim of this study was to estimate the incidence and costs of emergency department (ED) visits related to ADRs for patients greater than 65 years of age using administrative data, and to describe risk factors for experiencing severe ADRs.
Methods: We employed a retrospective cohort design based on population-based healthcare administrative clinical databases. Identification of ADR-related ED visits from the administrative database was based on International Classification of Diseases, 10th Revision-Canadian Enhancement (ICD-10-CA) codes for each ED visit. The incidence and costs of ADR-related ED visits and subsequent hospital admissions were estimated for all adults aged 66 years and above for the period April 2003-March 2008. Costs were standardized and reported in 2008 Canadian dollars. Logistic regression was used to detect risk factors for severe ADRs.
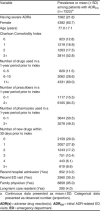
Results: Approximately 0.75% of total annual ED visits among adults aged 66 years and above were found to be ADR-related, and among these patients 21.6% were hospitalized. In 2007, the cost of ADR-related visits was $333 per ED visit and $7528 per hospitalization for a total annual cost of $13.6 million in Ontario, or an estimated $35.7 million in Canada. Severe ADRs were associated with sex, age, comorbid disease burden, multiple drugs, multiple pharmacies, newly prescribed drugs, recent ED visit, recent hospitalization and long-term care (LTC) residence.
Conclusions: ADRs are an important public health issue that threaten the safety of drug therapy and results in significant economic burden to the healthcare system. ED visits related to ADRs may be underestimated in retrospective studies using administrative data compared with prospective studies. Further research is needed to better understand the risk of experiencing severe ADRs among LTC residents.
Figures





References
-
- WHO International drug monitoring: the role of the national centers. World Health Organ Tech Rep Ser. 1972;498:1–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

