Antitumor activity of zoledronic acid in primary breast cancer cells determined by the ATP tumor chemosensitivity assay
- PMID: 22824103
- PMCID: PMC3416722
- DOI: 10.1186/1471-2407-12-308
Antitumor activity of zoledronic acid in primary breast cancer cells determined by the ATP tumor chemosensitivity assay
Abstract
Background: The NeoAzure study has demonstrated that the use of the bisphosphonate zoledronic acid (Zol) in the neoadjuvant setting increases the rate of complete response in primary breast cancer and therefore indicates direct antitumor activity. The purpose of this study was to compare the antitumor effect of Zol with standard chemotherapy in primary breast cancer cells using ATP-tumor chemosensitivity assay (ATP-TCA).
Methods: Breast cancer specimens were obtained from patients with breast cancer who underwent primary breast cancer surgery at the Department of Obstetrics and Gynecology, Tübingen, Germany, between 2006 through 2009. Antitumor effects of Zol, TAC (Docetaxel, Adriamycin, Cyclophosphamide) and FEC (5-Fluorouracil, Epirubicin, Cyclophosphamide) were tested in 116 fresh human primary breast cancer specimens using ATP-TCA. ATP-TCA results were analyzed with different cut-off levels for the half maximal inhibitory concentration (IC50), for IC90 and for the sensitivity index (IndexSUM). Each single agent or combination was tested at six doubling dilutions from 6.25, 12.5, 25, 50, 100, and 200% of test drug concentrations (TDC) derived from the plasma peak concentrations determined by pharmacokinetic data. The assay was carried out in duplicate wells with positive and negative controls.
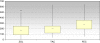
Results: The median IndexSUM value was lower for Zol than for the combined regimen FEC (36.8%) and TAC (12.9%), respectively, indicating increased antitumor activity of Zol in primary breast cancer cells. The difference regarding Zol and FEC was significant (p < 0.05). The median IC50 value for Zol (8.03% TDC) was significantly lower than the IC50 values for FEC (33.5% TDC) and TAC (19.3% TDC) treatment (p < 0.05). However, the median IC90 value for Zol (152.5% TDC) was significantly higher than the IC90 value obtained with TAC (49.5% TDC; p < 0.05), but similar to the IC90 value for FEC (180.9% TDC). In addition a significant positive correlation was observed for the IndexSum of Zol and the ER status (p < 0.01).
Conclusion: Zoledronic acid has a strong antitumor effect on primary breast cancer cells in vitro which is equal or superior to commonly used chemotherapeutic regimens for treating breast cancer.
Figures
Similar articles
-
Activity of mevalonate pathway inhibitors against breast and ovarian cancers in the ATP-based tumour chemosensitivity assay.BMC Cancer. 2009 Jan 28;9:38. doi: 10.1186/1471-2407-9-38. BMC Cancer. 2009. PMID: 19175937 Free PMC article.
-
Pilot studies of the effect of zoledronic acid (Zometa) on tumor-derived cells ex vivo in the ATP-based tumor chemosensitivity assay.Anticancer Drugs. 2005 Oct;16(9):969-76. doi: 10.1097/01.cad.0000176500.56057.66. Anticancer Drugs. 2005. PMID: 16162973
-
HER-2/neu overexpression and in vitro chemosensitivity to CMF and FEC in primary breast cancer.Breast Cancer Res Treat. 2001 Sep;69(1):53-63. doi: 10.1023/a:1012226006395. Breast Cancer Res Treat. 2001. PMID: 11759828
-
The new bisphosphonate, Zometa (zoledronic acid), decreases skeletal complications in both osteolytic and osteoblastic lesions: a comparison to pamidronate.Cancer Invest. 2002;20 Suppl 2:45-54. doi: 10.1081/cnv-120014886. Cancer Invest. 2002. PMID: 12442349 Review.
-
Zoledronic acid: an unending tale for an antiresorptive agent.Expert Opin Pharmacother. 2010 Jan;11(1):141-54. doi: 10.1517/14656560903485664. Expert Opin Pharmacother. 2010. PMID: 20001436 Review.
Cited by
-
Potential use of bisphosphonates in invasive extramammary Paget's disease: an immunohistochemical Investigation.Clin Dev Immunol. 2013;2013:164982. doi: 10.1155/2013/164982. Epub 2013 Mar 31. Clin Dev Immunol. 2013. PMID: 23606867 Free PMC article.
-
The miR-21/PTEN/Akt signaling pathway is involved in the anti-tumoral effects of zoledronic acid in human breast cancer cell lines.Naunyn Schmiedebergs Arch Pharmacol. 2016 May;389(5):529-38. doi: 10.1007/s00210-016-1224-8. Epub 2016 Feb 24. Naunyn Schmiedebergs Arch Pharmacol. 2016. PMID: 26905520
-
Purinergic signalling and cancer.Purinergic Signal. 2013 Dec;9(4):491-540. doi: 10.1007/s11302-013-9372-5. Purinergic Signal. 2013. PMID: 23797685 Free PMC article. Review.
-
Identification of proteins associated with paclitaxel resistance of epithelial ovarian cancer using iTRAQ-based proteomics.Oncol Lett. 2018 Jun;15(6):9793-9801. doi: 10.3892/ol.2018.8600. Epub 2018 Apr 27. Oncol Lett. 2018. PMID: 29928353 Free PMC article.
-
Randomized Controlled Trial of Zoledronic Acid plus Chemotherapy versus Chemotherapy Alone as Neoadjuvant Treatment of HER2-Negative Primary Breast Cancer (JONIE Study).PLoS One. 2015 Dec 3;10(12):e0143643. doi: 10.1371/journal.pone.0143643. eCollection 2015. PLoS One. 2015. PMID: 26633806 Free PMC article. Clinical Trial.
References
-
- Lipton A, Theriault RL, Hortobagyi GN, Simeone J, Knight RD, Mellars K, Reitsma DJ, Heffernan M, Seaman JJ. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer. 2000;88:1082–1090. doi: 10.1002/(SICI)1097-0142(20000301)88:5<1082::AID-CNCR20>3.0.CO;2-Z. - DOI - PubMed
-
- Gnant M, Mlineritsch B, Luschin-Ebengreuth G, Kainberger F, Kässmann H, Piswanger-Sölkner JC, Seifert M, Ploner F, Menzel C, Dubsky P, Fitzal F, Bjelic-Radisic V, Steger G, Greil R, Marth C, Kubista E, Samonigg H, Wohlmuth P, Mittlböck M, Jakesz R. Austrian Breast and Colorectal Cancer Study Group (ABCSG) Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with earlystage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol. 2008;9:840–849. doi: 10.1016/S1470-2045(08)70204-3. - DOI - PubMed
-
- Aapro M, Abrahamsson PA, Body JJ, Coleman RE, Colomer R, Costa L, Crinò L, Dirix L, Gnant M, Gralow J, Hadji P, Hortobagyi GN, Jonat W, Lipton A, Monnier A, Paterson AH, Rizzoli R, Saad F, Thürlimann B. Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Ann Oncol. 2008;19(3):420–432. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical