Clinical adoption of prognostic biomarkers: the case for heart failure
- PMID: 22824105
- PMCID: PMC3718550
- DOI: 10.1016/j.pcad.2012.05.004
Clinical adoption of prognostic biomarkers: the case for heart failure
Abstract
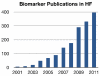
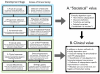
The recent explosion of scientific knowledge and technological progress has led to the discovery of a large array of circulating molecules commonly referred to as biomarkers. Biomarkers in heart failure (HF) research have been used to provide pathophysiologic insights, aid in establishing the diagnosis, refine prognosis, guide management, and target treatment. However, beyond diagnostic applications of natriuretic peptides, there are currently few widely recognized applications for biomarkers in HF. This represents a remarkable discordance considering the number of molecules that have been shown to correlate with outcomes, refine risk prediction, or track disease severity in HF in the past decade. In this article, we use a broad framework proposed for cardiovascular risk markers to summarize the current state of biomarker development for patients with HF. We use this framework to identify the challenges of biomarker adoption for risk prediction, disease management, and treatment selection for HF and suggest considerations for future research.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Heart failure biomarkers.J Cardiovasc Transl Res. 2013 Aug;6(4):471-84. doi: 10.1007/s12265-013-9465-0. Epub 2013 Apr 20. J Cardiovasc Transl Res. 2013. PMID: 23604646 Review.
-
Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure: A Scientific Statement From the American Heart Association.Circulation. 2017 May 30;135(22):e1054-e1091. doi: 10.1161/CIR.0000000000000490. Epub 2017 Apr 26. Circulation. 2017. PMID: 28446515 Review.
-
Biomarkers in heart failure: a focus on natriuretic peptides.Heart. 2024 May 10;110(11):809-818. doi: 10.1136/heartjnl-2020-318553. Heart. 2024. PMID: 37673654 Review.
-
Galectin-3: a new biomarker for the diagnosis, analysis and prognosis of acute and chronic heart failure.Arch Cardiovasc Dis. 2013 Oct;106(10):541-6. doi: 10.1016/j.acvd.2013.06.054. Epub 2013 Oct 3. Arch Cardiovasc Dis. 2013. PMID: 24090952
-
Biomarkers of inflammation and cardiac remodeling: the quest of relevant companions for the risk stratification of heart failure patients is still ongoing.Biochem Med (Zagreb). 2011;21(3):254-63. doi: 10.11613/bm.2011.035. Biochem Med (Zagreb). 2011. PMID: 22420239 Review.
Cited by
-
miR-129-5p improves cardiac function in rats with chronic heart failure through targeting HMGB1.Mamm Genome. 2019 Oct;30(9-10):276-288. doi: 10.1007/s00335-019-09817-0. Epub 2019 Oct 23. Mamm Genome. 2019. PMID: 31646380
-
Beyond the Limits: Clinical Utility of Novel Cardiac Biomarkers.Biomed Res Int. 2015;2015:187384. doi: 10.1155/2015/187384. Epub 2015 Oct 4. Biomed Res Int. 2015. PMID: 26504786 Free PMC article. Review.
-
Increased Plasma Concentrations of Extracellular Vesicles Are Associated with Pro-Inflammatory and Pro-Thrombotic Characteristics of Left and Right Ventricle Mechanical Support Devices.J Cardiovasc Dev Dis. 2023 Jan 5;10(1):21. doi: 10.3390/jcdd10010021. J Cardiovasc Dev Dis. 2023. PMID: 36661916 Free PMC article.
-
Ischemic biomarker heart-type fatty acid binding protein (hFABP) in acute heart failure - diagnostic and prognostic insights compared to NT-proBNP and troponin I.BMC Cardiovasc Disord. 2015 Jun 14;15:50. doi: 10.1186/s12872-015-0026-0. BMC Cardiovasc Disord. 2015. PMID: 26072112 Free PMC article.
-
The Indian Consensus Document on cardiac biomarker.Indian Heart J. 2014 Jan-Feb;66(1):73-82. doi: 10.1016/j.ihj.2013.12.053. Indian Heart J. 2014. PMID: 24581100 Free PMC article. Review.
References
-
- Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. - PubMed
-
- Barth AS, Chakir K, Kass DA, Tomaselli GF. Transcriptome, Proteome, and Metabolome in Dyssynchronous Heart Failure and CRT. J Cardiovasc Transl Res. 2012;5:180–7. - PubMed
-
- Tang WHW, Francis GS, Morrow DA, et al. National Academy of Clinical Biochemistry Laboratory Medicine practice guidelines: Clinical utilization of cardiac biomarker testing in heart failure. Circulation. 2007;116:e99–109. - PubMed
-
- Morrow DA, de Lemos JA. Benchmarks for the assessment of novel cardiovascular biomarkers. Circulation. 2007;115:949–52. - PubMed
-
- Maisel A. Biomonitoring and biomarker-guided therapy: the next step in heart failure and biomarker research. J Am Coll Cardiol. 2011;58:1890–2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

