Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study
- PMID: 22824372
- PMCID: PMC3419089
- DOI: 10.1186/1742-2094-9-179
Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study
Abstract
Background: This study undertakes a systematic and comprehensive analysis of brain gene expression profiles of immune/inflammation-related genes in aging and Alzheimer's disease (AD).
Methods: In a well-powered microarray study of young (20 to 59 years), aged (60 to 99 years), and AD (74 to 95 years) cases, gene responses were assessed in the hippocampus, entorhinal cortex, superior frontal gyrus, and post-central gyrus.
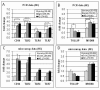
Results: Several novel concepts emerge. First, immune/inflammation-related genes showed major changes in gene expression over the course of cognitively normal aging, with the extent of gene response far greater in aging than in AD. Of the 759 immune-related probesets interrogated on the microarray, approximately 40% were significantly altered in the SFG, PCG and HC with increasing age, with the majority upregulated (64 to 86%). In contrast, far fewer immune/inflammation genes were significantly changed in the transition to AD (approximately 6% of immune-related probesets), with gene responses primarily restricted to the SFG and HC. Second, relatively few significant changes in immune/inflammation genes were detected in the EC either in aging or AD, although many genes in the EC showed similar trends in responses as in the other brain regions. Third, immune/inflammation genes undergo gender-specific patterns of response in aging and AD, with the most pronounced differences emerging in aging. Finally, there was widespread upregulation of genes reflecting activation of microglia and perivascular macrophages in the aging brain, coupled with a downregulation of select factors (TOLLIP, fractalkine) that when present curtail microglial/macrophage activation. Notably, essentially all pathways of the innate immune system were upregulated in aging, including numerous complement components, genes involved in toll-like receptor signaling and inflammasome signaling, as well as genes coding for immunoglobulin (Fc) receptors and human leukocyte antigens I and II.
Conclusions: Unexpectedly, the extent of innate immune gene upregulation in AD was modest relative to the robust response apparent in the aged brain, consistent with the emerging idea of a critical involvement of inflammation in the earliest stages, perhaps even in the preclinical stage, of AD. Ultimately, our data suggest that an important strategy to maintain cognitive health and resilience involves reducing chronic innate immune activation that should be initiated in late midlife.
Figures



References
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I. et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/S0197-4580(00)00124-X. - DOI - PMC - PubMed
-
- McGeer PL, Rogers J, McGeer EG. Inflammation, anti-inflammatory agents and Alzheimer disease: the last 12 years. J Alzheimers Dis. 2006;9:271–276. - PubMed
-
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

