Flexibility of interval between vaccinations with AS03A-adjuvanted influenza A (H1N1) 2009 vaccine in adults aged 18-60 and >60 years: a randomized trial
- PMID: 22824474
- PMCID: PMC3522029
- DOI: 10.1186/1471-2334-12-162
Flexibility of interval between vaccinations with AS03A-adjuvanted influenza A (H1N1) 2009 vaccine in adults aged 18-60 and >60 years: a randomized trial
Abstract
Background: Flexibility of vaccination schedule and lower antigen content can facilitate pandemic vaccine coverage. We assessed the immune response and safety of AS03-adjuvanted A/California/7/2009 H1N1 pandemic vaccine containing half of the registered adult haemagglutinin (HA) antigen content, administered as a two-dose schedule at intervals of 21 days or 6 months in both young and elderly adults.
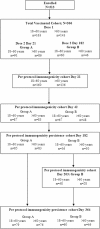
Methods: In this open-label randomized trial, healthy adults aged 18-60 years (N = 163) and >60 years (N = 143) received AS03A-adjuvanted A/California/7/2009 H1N1 vaccine containing 1.9 μg HA on Day 0. A second dose was given on Day 21 (n = 177) or Day 182 (n = 106). Haemagglutination-inhibition (HI) antibody responses were analyzed on Days 0, 21, 42, 182, 364 and additionally on Day 203 for subjects vaccinated on Day 182. Solicited and unsolicited adverse events were recorded.
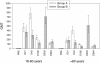
Results: The HI antibody response in both age strata 21 days after the first dose met and exceeded all regulatory acceptance criteria although the results suggested a lower response in the older age stratum (geometric mean titres [GMTs] for HI antibodies of 420.5 for subjects aged 18-60 years and 174.4 for those >60 years). A second dose of AS03A adjuvanted A/H1N1/2009 vaccine induced a further increase in antibody titres and the response was similar whether the second dose was administered at 21 days (GMTs of 771.8 for 18-60 years and 400.9 for >60 years) or 6 months (GMTs of 708.3 for 18-60 years and 512.1 for >60 years) following the first dose. Seroprotection rates remained high at 6 months after one dose or two doses while at 12 months rates tended to be higher for the 6 month interval schedule (93.3% for 18-60 years and 80.4% for >60 years) than the 21 day schedule (82.3% for 18-60 years and 50.0% for >60 years). Reactogenicity/safety profiles were similar for both schedules, there was no evidence of an increase in reactogenicity following the second dose.
Conclusions: The results indicate that flexibility in the dosing interval for AS03A adjuvanted vaccine may be possible. Such flexibility could help to reduce the logistic stress on delivery of pandemic vaccination programmes.
Trial registration: ClinicalTrials.gov, NCT00975884.
Figures
References
-
- Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, Bresee JS, Cox NJ. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR. 2009;58(RR-8):1–52. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

