Receptor-targeted therapy of human experimental urinary bladder cancers with cytotoxic LH-RH analog AN-152 [AEZS- 108]
- PMID: 22824624
- PMCID: PMC3443252
- DOI: 10.18632/oncotarget.546
Receptor-targeted therapy of human experimental urinary bladder cancers with cytotoxic LH-RH analog AN-152 [AEZS- 108]
Abstract
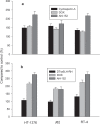
Many bladder cancers progress to invasion with poor prognosis; new therapeutic methods are needed. We developed a cytotoxic LH-RH analog, AN-152 (AEZS-108) containing doxorubicin (DOX), for targeted therapy of cancers expressing LHRH receptors. We investigated the expression of LH-RH receptors in clinical bladder cancers and in HT-1376, J82, RT-4 and HT-1197 human bladder cancer lines. The effect of analog, AN-152, on growth of these tumor lines xenografted into nude mice was analyzed. Using molecular and functional assays, we also evaluated the differences between the effects of AN-152, and DOX alone. We demonstrated the expression of LH-RH receptors on 18 clinical bladder cancers by immunohistochemistry and on four human urinary bladder cancer lines HT-1376, J82, RT-4 and HT-1197 by Western blotting and binding assays. AN-152 powerfully inhibited growth of these bladder cancers in nude mice. AN-152 exerted greater effects than DOX and was less toxic. DOX activated strong multidrug resistance mechanisms in RT-4 and HT-1197 cancers, while AN-152 had no or less such effect. PCR assays and in vitro studies revealed differences in the action of AN-152 and DOX on the expression of genes involved in apoptosis. These results suggest that targeted cytotoxic LH-RH analog, AN-152 (AEZS- 108), should be examined for treatment of patients with LH-RH receptor positive invasive bladder cancers.
Conflict of interest statement
Dr. A.V. Schally is listed as co-inventor on the Tulane University patents on AN-152, but this study was experimental. No potential conflict of interest exists for other authors.
Figures





References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. - PubMed
-
- Yagoda A. Progress in Cancer Reseach and Therapy. Raven Press; New York: Chemotherapy of advanced bladder cancer; pp. 251–260.
-
- Citrin DL, Hogan TF, Davis TE. A study of cyclophosphamide, adriamycin, cis-platinum, and methotrexate in advanced transitional cell carcinoma of the urinary tract. Cancer. 1983;51(1):1–4. - PubMed
-
- Loehrer PJ, Sr., Einhorn LH, Elson PJ, Crawford ED, Kuebler P, Tannock I, Raghavan D, Stuart-Harris R, Sarosdy MF, Lowe BA, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1992;10(7):1066–1073. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

