Guided desaturation of unactivated aliphatics
- PMID: 22824894
- PMCID: PMC3405363
- DOI: 10.1038/nchem.1385
Guided desaturation of unactivated aliphatics
Abstract
The excision of hydrogen from an aliphatic carbon chain to produce an isolated olefin (desaturation) without overoxidation is one of the most impressive and powerful biosynthetic transformations for which there are no simple and mild laboratory substitutes. The versatility of olefins and the range of reactions they undergo are unsurpassed in functional group space. Thus, the conversion of a relatively inert aliphatic system into its unsaturated counterpart could open new possibilities in retrosynthesis. In this article, the invention of a directing group to achieve such a transformation under mild, operationally simple, metal-free conditions is outlined. This 'portable desaturase' (Tz(o)Cl) is a bench-stable, commercial entity (Aldrich, catalogue number L510092) that is facile to install on alcohol and amine functionalities to ultimately effect remote desaturation, while leaving behind a synthetically useful tosyl group.
Figures
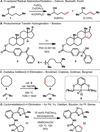



References
-
- Larock RC. Comprehensive Organic Transformation. New York: Wiley; 1999.
-
- Choi J, MacArthur AHR, Brookhart M, Goldman AS. Dehydrogenation and Related Reactions Catalyzed by Iridium Pincer Complexes. Chem. Rev. 2011;111:1761–1779. - PubMed
-
- Breslow R, et al. Remote oxidation of steroids by photolysis of attached benzophenone groups. J. Am. Chem. Soc. 1973;95:3251–3262. - PubMed
-
- Breslow R. Biomimetic Chemistry and Artificial Enzymes: Catalysis by Design. Acc. Chem. Res. 1995;28:146–153.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

