TAZ induces growth factor-independent proliferation through activation of EGFR ligand amphiregulin
- PMID: 22825057
- PMCID: PMC3419062
- DOI: 10.4161/cc.21386
TAZ induces growth factor-independent proliferation through activation of EGFR ligand amphiregulin
Abstract
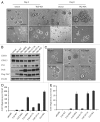
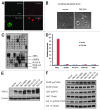
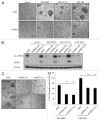
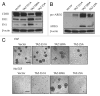
The Hippo signaling pathway regulates cellular proliferation and survival, thus exerting profound effects on normal cell fate and tumorigenesis. We previously showed that the pivotal effector of this pathway, YAP, is amplified in tumors and promotes epithelial-to-mesenchymal transition (EMT) and malignant transformation. Here, we report that overexpression of TAZ, a paralog of YAP, in human mammary epithelial cells promotes EMT and, in particular, some invasive structures in 3D cultures. TAZ also leads to cell migration and anchorage-independent growth in soft agar. Furthermore, we identified amphiregulin (AREG), an epidermal growth factor receptor (EGFR) ligand, as a target of TAZ. We show that AREG functions in a non-cell-autonomous manner to mediate EGF-independent growth and malignant behavior of mammary epithelial cells. In addition, ablation of TEAD binding completely abolishes the TAZ-induced phenotype. Last, analysis of breast cancer patient samples reveals a positive correlation between TAZ and AREG in vivo. In summary, TAZ-dependent secretion of AREG indicates that activation of the EGFR signaling is an important non-cell-autonomous effector of the Hippo pathway, and TAZ as well as its targets may play significant roles in breast tumorigenesis and metastasis.
Figures

Similar articles
-
YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway.Nat Cell Biol. 2009 Dec;11(12):1444-50. doi: 10.1038/ncb1993. Epub 2009 Nov 22. Nat Cell Biol. 2009. PMID: 19935651 Free PMC article.
-
Mammary tumorigenesis induced by fibroblast growth factor receptor 1 requires activation of the epidermal growth factor receptor.J Cell Sci. 2011 Sep 15;124(Pt 18):3106-17. doi: 10.1242/jcs.082651. Epub 2011 Aug 24. J Cell Sci. 2011. PMID: 21868365 Free PMC article.
-
Reversible interconversion and maintenance of mammary epithelial cell characteristics by the ligand-regulated EGFR system.Sci Rep. 2016 Feb 2;6:20209. doi: 10.1038/srep20209. Sci Rep. 2016. PMID: 26831618 Free PMC article.
-
The ADAM17-amphiregulin-EGFR axis in mammary development and cancer.J Mammary Gland Biol Neoplasia. 2008 Jun;13(2):181-94. doi: 10.1007/s10911-008-9084-6. Epub 2008 May 10. J Mammary Gland Biol Neoplasia. 2008. PMID: 18470483 Free PMC article. Review.
-
Amphiregulin: role in mammary gland development and breast cancer.J Mammary Gland Biol Neoplasia. 2008 Jun;13(2):159-69. doi: 10.1007/s10911-008-9075-7. Epub 2008 Apr 9. J Mammary Gland Biol Neoplasia. 2008. PMID: 18398673 Review.
Cited by
-
The roles of signaling pathways in epithelial-to-mesenchymal transition of PVR.Mol Vis. 2015 Jun 24;21:706-10. eCollection 2015. Mol Vis. 2015. PMID: 26109834 Free PMC article. Review.
-
Harmine induces anticancer activity in breast cancer cells via targeting TAZ.Int J Oncol. 2019 Jun;54(6):1995-2004. doi: 10.3892/ijo.2019.4777. Epub 2019 Apr 9. Int J Oncol. 2019. PMID: 31081045 Free PMC article.
-
The Hippo Signaling Transducer TAZ Regulates Mammary Gland Morphogenesis and Carcinogen-induced Mammary Tumorigenesis.Sci Rep. 2018 Apr 24;8(1):6449. doi: 10.1038/s41598-018-24712-5. Sci Rep. 2018. PMID: 29691438 Free PMC article.
-
Mutant p53 Protein and the Hippo Transducers YAP and TAZ: A Critical Oncogenic Node in Human Cancers.Int J Mol Sci. 2017 May 3;18(5):961. doi: 10.3390/ijms18050961. Int J Mol Sci. 2017. PMID: 28467351 Free PMC article. Review.
-
YAP forms autocrine loops with the ERBB pathway to regulate ovarian cancer initiation and progression.Oncogene. 2015 Dec 10;34(50):6040-54. doi: 10.1038/onc.2015.52. Epub 2015 Mar 23. Oncogene. 2015. PMID: 25798835 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
