DNA excision repair: where do all the dimers go?
- PMID: 22825251
- PMCID: PMC3442910
- DOI: 10.4161/cc.21126
DNA excision repair: where do all the dimers go?
Abstract
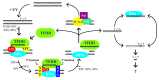
Exposure of cells to UV light from the sun causes the formation of pyrimidine dimers in DNA that have the potential to lead to mutation and cancer. In humans, pyrimidine dimers are removed from the genome in the form of ~30 nt-long oligomers by concerted dual incisions. Though nearly 50 y of excision repair research has uncovered many details of UV photoproduct damage recognition and removal, the fate of the excised oligonucleotides and, in particular, the ultimate fate of the chemically very stable pyrimidine dimers remain unknown. Physiologically relevant UV doses introduce hundreds of thousands of pyrimidine dimers in diploid human cells, which are excised from the genome within ~24 h. Once removed from the genome, "where do all the dimers go?" In a recent study we addressed this question. Although our study did not determine the fate of the dimer itself, it revealed that the excised ~30-mer is released from the duplex in a tight complex with the transcription/repair factor TFIIH. This finding combined with recent reports that base and oligonucleotide products of the base and double-strand break repair pathways also make stable complexes with the cognate repair enzymes, and that these complexes activate the MAP kinase and checkpoint signaling pathways, respectively, raises the possibility that TFIIH-30-mer excision complexes may play a role in signaling reactions in response to UV damage.
Figures


Comment in
-
DNA nucleotide excision repair, where do all the cyclobutane pyrimidine dimers go?Cell Cycle. 2013 May 15;12(10):1642. doi: 10.4161/cc.24701. Epub 2013 Apr 19. Cell Cycle. 2013. PMID: 23603991 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
