Protein A-specific monoclonal antibodies and prevention of Staphylococcus aureus disease in mice
- PMID: 22825452
- PMCID: PMC3457578
- DOI: 10.1128/IAI.00230-12
Protein A-specific monoclonal antibodies and prevention of Staphylococcus aureus disease in mice
Abstract
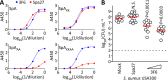
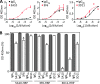
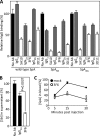
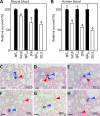
Staphylococcus aureus is a leading cause of human soft tissue infections and bacterial sepsis. The emergence of antibiotic-resistant strains (methicillin-resistant S. aureus [MRSA]) has prompted research into staphylococcal vaccines and preventive measures. The envelope of S. aureus is decorated with staphylococcal protein A (SpA), which captures the Fcγ portion of immunoglobulins to prevent opsonophagocytosis and associates with the Fab portion of V(H)3-type B cell receptors to trigger B cell superantigen activity. Nontoxigenic protein A (SpA(KKAA)), when used as an immunogen in mice, stimulates humoral immune responses that neutralize the Fcγ and the V(H)3(+) Fab binding activities of SpA and provide protection from staphylococcal abscess formation in mice. Here, we isolated monoclonal antibodies (MAbs) against SpA(KKAA) that, by binding to the triple-helical bundle fold of its immunoglobulin binding domains (IgBDs), neutralize the Fcγ and Fab binding activities of SpA. SpA(KKAA) MAbs promoted opsonophagocytic killing of MRSA in mouse and human blood, provided protection from abscess formation, and stimulated pathogen-specific immune responses in a mouse model of staphylococcal disease. Thus, SpA(KKAA) MAbs may be useful for the prevention and therapy of staphylococcal disease in humans.
Figures



References
-
- Atkins KL, et al. 2008. S. aureus IgG-binding proteins SpA and Sbi: host specificity and mechanisms of immune complex formation. Mol. Immunol. 45:1600–1611 - PubMed
-
- Baba T, et al. 2002. Genome and virulence determinants of high-virulence community acquired MRSA. Lancet 359:1819–1827 - PubMed
-
- Berberian L, Goodglick L, Kipps TJ, Braun J. 1993. Immunoglobulin VH3 gene products: natural ligands for HIV gp120. Science 261:1588–1591 - PubMed
-
- Berberian L, Valles-Ayoub Y, Sun N, Martinez-Maza O, Braun J. 1991. A VH clonal deficit in human immunodeficiency virus-positive individuals reflects a B-cell maturational arrest. Blood 78:175–179 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

