Alpha-synuclein: from secretion to dysfunction and death
- PMID: 22825468
- PMCID: PMC3406593
- DOI: 10.1038/cddis.2012.94
Alpha-synuclein: from secretion to dysfunction and death
Abstract
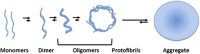
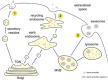
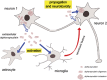
The aggregation, deposition, and dysfunction of alpha-synuclein (aSyn) are common events in neurodegenerative disorders known as synucleinopathies. These include Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy. A growing body of knowledge on the biology of aSyn is emerging and enabling novel hypotheses to be tested. In particular, the hypothesis that aSyn is secreted from neurons, thus contributing to the spreading of pathology not only in the brain but also in other organs, is gaining momentum. Nevertheless, the precise mechanism(s) of secretion, as well as the consequences of extracellular aSyn species for neighboring cells are still unclear. Here, we review the current literature and integrate existing data in order to propose possible mechanisms of secretion, cell dysfunction, and death. Ultimately, the complete understanding of these processes might open novel avenues for the development of new therapeutic strategies.
Figures
References
-
- Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9449. - PubMed
-
- Jo E, McLaurin J, Yip CM, George-Hyslop P, Fraser PE. alpha-Synuclein membrane interactions and lipid specificity. J Biol Chem. 2000;275:34328–34334. - PubMed
-
- Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994;345:27–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

