Generation of N-acylphosphatidylethanolamine by members of the phospholipase A/acyltransferase (PLA/AT) family
- PMID: 22825852
- PMCID: PMC3442523
- DOI: 10.1074/jbc.M112.368712
Generation of N-acylphosphatidylethanolamine by members of the phospholipase A/acyltransferase (PLA/AT) family
Abstract
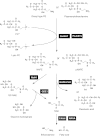
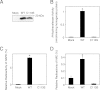
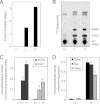
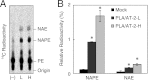
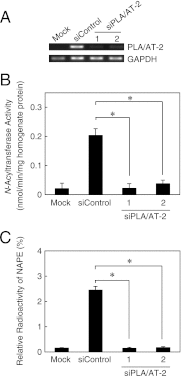
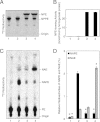
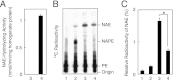
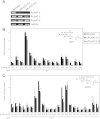
Bioactive N-acylethanolamines (NAEs), including N-palmitoylethanolamine, N-oleoylethanolamine, and N-arachidonoylethanolamine (anandamide), are formed from membrane glycerophospholipids in animal tissues. The pathway is initiated by N-acylation of phosphatidylethanolamine to form N-acylphosphatidylethanolamine (NAPE). Despite the physiological importance of this reaction, the enzyme responsible, N-acyltransferase, remains molecularly uncharacterized. We recently demonstrated that all five members of the HRAS-like suppressor tumor family are phospholipid-metabolizing enzymes with N-acyltransferase activity and are renamed HRASLS1-5 as phospholipase A/acyltransferase (PLA/AT)-1-5. However, it was poorly understood whether these proteins were involved in the formation of NAPE in living cells. In the present studies, we first show that COS-7 cells transiently expressing recombinant PLA/AT-1, -2, -4, or -5, and HEK293 cells stably expressing PLA/AT-2 generated significant amounts of [(14)C]NAPE and [(14)C]NAE when cells were metabolically labeled with [(14)C]ethanolamine. Second, as analyzed by liquid chromatography-tandem mass spectrometry, the stable expression of PLA/AT-2 in cells remarkably increased endogenous levels of NAPEs and NAEs with various N-acyl species. Third, when NAPE-hydrolyzing phospholipase D was additionally expressed in PLA/AT-2-expressing cells, accumulating NAPE was efficiently converted to NAE. We also found that PLA/AT-2 was partly responsible for NAPE formation in HeLa cells that endogenously express PLA/AT-2. These results suggest that PLA/AT family proteins may produce NAPEs serving as precursors of bioactive NAEs in vivo.
Figures



References
-
- Schmid H. H. O., Schmid P. C., Natarajan V. (1990) N-Acylated glycerophospholipids and their derivatives. Prog. Lipid Res. 29, 1–43 - PubMed
-
- Hansen H. S., Moesgaard B., Hansen H. H., Petersen G. (2000) N-Acylethanolamines and precursor phospholipids. Relation to cell injury. Chem. Phys. Lipids 108, 135–150 - PubMed
-
- Devane W. A., Hanus L., Breuer A., Pertwee R. G., Stevenson L. A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258, 1946–1949 - PubMed
-
- Zygmunt P. M., Petersson J., Andersson D. A., Chuang H., Sørgård M., Di Marzo V., Julius D., Högestätt E. D. (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400, 452–457 - PubMed
-
- Calignano A., La Rana G., Giuffrida A., Piomelli D. (1998) Control of pain initiation by endogenous cannabinoids. Nature 394, 277–281 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

