Discovery of O-GlcNAc-6-phosphate modified proteins in large-scale phosphoproteomics data
- PMID: 22826440
- PMCID: PMC3494138
- DOI: 10.1074/mcp.M112.019760
Discovery of O-GlcNAc-6-phosphate modified proteins in large-scale phosphoproteomics data
Abstract
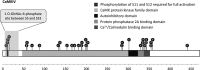
Phosphorylated O-GlcNAc is a novel post-translational modification that has so far only been found on the neuronal protein AP180 from the rat (Graham et al., J. Proteome Res. 2011, 10, 2725-2733). Upon collision induced dissociation, the modification generates a highly mass deficient fragment ion (m/z 284.0530) that can be used as a reporter for the identification of phosphorylated O-GlcNAc. Using a publically available mouse brain phosphoproteome data set, we employed our recently developed Oscore software to re-evaluate high resolution/high accuracy tandem mass spectra and discovered the modification on 23 peptides corresponding to 11 mouse proteins. The systematic analysis of 220 candidate phosphoGlcNAc tandem mass spectra as well as a synthetic standard enabled the dissection of the major phosphoGlcNAc fragmentation pathways, suggesting that the modification is O-GlcNAc-6-phosphate. We find that the classical O-GlcNAc modification often exists on the same peptides indicating that O-GlcNAc-6-phosphate may biosynthetically arise in two steps involving the O-GlcNAc transferase and a currently unknown kinase. Many of the identified proteins are involved in synaptic transmission and for Ca(2+)/calmodulin kinase IV, the O-GlcNAc-6-phosphate modification was found in the vicinity of two autophosphorylation sites required for full activation of the kinase suggesting a potential regulatory role for O-GlcNAc-6-phosphate. By re-analyzing mass spectrometric data from human embryonic and induced pluripotent stem cells, our study also identified Zinc finger protein 462 (ZNF462) as the first human O-GlcNAc-6-phosphate modified protein. Collectively, the data suggests that O-GlcNAc-6-phosphate is a general post-translation modification of mammalian proteins with a variety of possible cellular functions.
Figures


References
-
- Hart G. W., Housley M. P., Slawson C. (2007) Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446, 1017–1022 - PubMed
-
- Hu P., Shimoji S., Hart G. W. Site-specific interplay between O-GlcNAcylation and phosphorylation in cellular regulation. FEBS Lett. 584, 2526–2538 - PubMed
-
- Yang W. H., Kim J. E., Nam H. W., Ju J. W., Kim H. S., Kim Y. S., Cho J. W. (2006) Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat. Cell Biol. 8, 1074–1083 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

