Autoimmunity in wiskott-Aldrich syndrome: an unsolved enigma
- PMID: 22826711
- PMCID: PMC3399097
- DOI: 10.3389/fimmu.2012.00209
Autoimmunity in wiskott-Aldrich syndrome: an unsolved enigma
Abstract
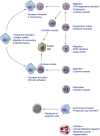
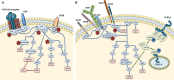
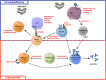
Wiskott-Aldrich Syndrome (WAS) is a severe X-linked Primary Immunodeficiency that affects 1-10 out of 1 million male individuals. WAS is caused by mutations in the WAS Protein (WASP) expressing gene that leads to the absent or reduced expression of the protein. WASP is a cytoplasmic protein that regulates the formation of actin filaments in hematopoietic cells. WASP deficiency causes many immune cell defects both in humans and in the WAS murine model, the Was(-/-) mouse. Both cellular and humoral immune defects in WAS patients contribute to the onset of severe clinical manifestations, in particular microthrombocytopenia, eczema, recurrent infections, and a high susceptibility to develop autoimmunity and malignancies. Autoimmune diseases affect from 22 to 72% of WAS patients and the most common manifestation is autoimmune hemolytic anemia, followed by vasculitis, arthritis, neutropenia, inflammatory bowel disease, and IgA nephropathy. Many groups have widely explored immune cell functionality in WAS partially explaining how cellular defects may lead to pathology. However, the mechanisms underlying the occurrence of autoimmune manifestations have not been clearly described yet. In the present review, we report the most recent progresses in the study of immune cell function in WAS that have started to unveil the mechanisms contributing to autoimmune complications in WAS patients.
Keywords: B lymphocytes; T lymphocytes; Wiskott–Aldrich syndrome; autoimmunity; primary immunodeficiency.
Figures

References
-
- Albert M. H., Bittner T. C., Nonoyama S., Notarangelo L. D., Burns S., Imai K., Espanol T., Fasth A., Pellier I., Strauss G., Morio T., Gathmann B., Noordzij J. G., Fillat C., Hoenig M., Nathrath M., Meindl A., Pagel P., Wintergerst U., Fischer A., Thrasher A. J., Belohradsky B. H., Ochs H. D. (2010). X-linked thrombocytopenia (XLT) due to WAS mutations: clinical characteristics, long-term outcome, and treatment options. Blood 115, 3231–323810.1182/blood-2009-09-239087 - DOI - PubMed
-
- Antoine C., Muller S., Cant A., Cavazzana-Calvo M., Veys P., Vossen J., Fasth A., Heilmann C., Wulffraat N., Seger R., Blanche S., Friedrich W., Abinun M., Davies G., Bredius R., Schulz A., Landais P., Fischer A. (2003). Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968-99. Lancet 361, 553–56010.1016/S0140-6736(03)12513-5 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

