Aromatic interactions as control elements in stereoselective organic reactions
- PMID: 22827883
- PMCID: PMC3495095
- DOI: 10.1021/ar3000794
Aromatic interactions as control elements in stereoselective organic reactions
Abstract
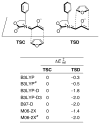
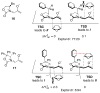
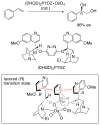
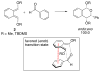
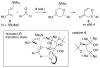
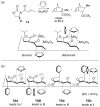
This Account describes how attractive interactions of aromatic rings with other groups can influence and control the stereoselectivity of many reactions. Recent developments in theory have improved the accuracy in the modeling of aromatic interactions. Quantum mechanical modeling can now provide insights into the roles of these interactions at a level of detail not previously accessible, both for ground-state species and for transition states of chemical reactions. In this Account, we show how transition-state modeling led to the discovery of the influence of aryl groups on the stereoselectivities of several types of organic reactions, including asymmetric dihydroxylations, transfer hydrogenations, hetero-Diels-Alder reactions, acyl transfers, and Claisen rearrangements. Our recent studies have also led to a novel mechanistic picture for two classes of (4 + 3) cycloadditions, both of which involve reactions of furans with oxyallyl intermediates. The first class of cycloadditions, developed by Hsung, features neutral oxyallyl intermediates that contain a chiral oxazolidinone auxiliary. Originally, it was thought that these cycloadditions relied on differential steric crowding of the two faces of a planar intermediate. Computations reveal a different picture and show that cycloaddition with furan takes place preferentially through the more crowded transition state: the furan adds on the same side as the Ph substituent of the oxazolidinone. The crowded transition state is stabilized by a CH-π interaction between furan and Ph worth approximately 2 kcal/mol. Attractive interactions with aromatic rings also control the stereoselectivity in a second class of (4+3) cycloadditions involving chiral alkoxy siloxyallyl cations. Alkoxy groups derived from chiral α-methylbenzyl alcohols favor crowded transition states, where a stabilizing CH-π interaction is present between the furan and the Ar group. The cationic cycloadditions are stepwise, while the Hsung cycloadditions are concerted. Our results suggest that this form of CH- π-directed stereocontrol is quite general and likely controls the stereoselectivities of other addition reactions in which one face of a planar intermediate bears a pendant aromatic substituent.
Figures










References
-
-
See, for example: Nishio M. The CH/π hydrogen bond in chemistry. Conformation, supramolecules, optical resolution and interactions involving carbohydrates. Phys Chem Chem Phys. 2011;13:13873–13900.Lehn J-M. Supramolecular Chemistry: Concepts and Perspectives. VCH; Weinheim: 1995. Gillard RE, Raymo FM, Stoddart JF. Controlling Self-Assembly. Chem Eur J. 1997;3:1933–1940.Meyer EA, Castellano RK, Diederich F. Interactions with Aromatic Rings in Chemical and Biological Recognition. Angew Chem Int Ed. 2003;42:1210–1250.Burley SK, Petsko GA. Aromatic-Aromatic Interaction: A Mechanism of Protein Structure Stabilization. Science. 1985;229:23–28.Waters ML. Aromatic Interactions in Peptides. Biopolymers, Peptide Science. 2004;76:435–445.
-
-
-
For reviews, see: Nishio M, Hirota M, Umezawa Y. The CH/π Interaction: Evidence, Nature, and Consequences. Wiley-VCH; New York: 1998. Jones GB. π Shielding in organic synthesis. Tetrahedron. 2001;57:7999–8016.Nishio M. CH/π hydrogen bonds in organic reactions. Tetrahedron. 2005;61:6923–6950.
-
-
-
For example, see: Corey EJ, Matsumura Y. Evidence for the Importance of π-π-Attractive Interactions in Enantioselective Diels–Alder Reactions Chiral Catalysts of Type (RO)2TiCl2. Tetrahedron Lett. 1991;32:6289–6292.Ishihara K, Kurihara H, Matsumoto M, Yamamoto H. Design of Brønsted Acid-Assisted Chiral Lewis Acid (BLA) Catalysts for Highly Enantioselective Diels–Alder Reactions. J Am Chem Soc. 1998;120:6920–6930.Kürti L, Blewett MM, Corey EJ. Origin of Enantioselectivity in the Jacobsen Epoxidation of Olefins. Org Lett. 2009;11:4592–4595.
-
-
- Johnson ER, Mackie ID, DiLabio GA. Dispersion interactions in density-functional theory. J Phys Org Chem. 2009;22:1127–1135.
- Sherrill CD. Computations of Noncovalent π Interactions. In: Lipkowitz KB, Cundari TR, editors. Reviews in Computational Chemistry. Vol. 26. Wiley; New York: 2009. pp. 1–38.
- Grimme S. Density functional theory with London dispersion corrections. WIREs Comp Mol Sci. 2011;1:211–228.
-
-
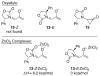
Hartree–Fock (HF) theory fails to predict dispersion binding, because it neglects dynamical correlation.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

