Dynamic force spectroscopy on supported lipid bilayers: effect of temperature and sample preparation
- PMID: 22828330
- PMCID: PMC3388201
- DOI: 10.1016/j.bpj.2012.05.039
Dynamic force spectroscopy on supported lipid bilayers: effect of temperature and sample preparation
Abstract
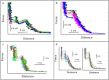
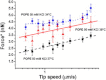
Biological membranes are constantly exposed to forces. The stress-strain relation in membranes determines the behavior of many integral membrane proteins or other membrane related-proteins that show a mechanosensitive behavior. Here, we studied by force spectroscopy the behavior of supported lipid bilayers (SLBs) subjected to forces perpendicular to their plane. We measured the lipid bilayer mechanical properties and the force required for the punch-through event characteristic of atomic force spectroscopy on SLBs as a function of the interleaflet coupling. We found that for an uncoupled bilayer, the overall tip penetration occurs sequentially through the two leaflets, giving rise to two penetration events. In the case of a bilayer with coupled leaflets, penetration of the atomic force microscope tip always occurred in a single step. Considering the dependence of the jump-through force value on the tip speed, we also studied the process in the context of dynamic force spectroscopy (DFS). We performed DFS experiments by changing the temperature and cantilever spring constant, and analyzed the results in the context of the developed theories for DFS. We found that experiments performed at different temperatures and with different cantilever spring constants enabled a more effective comparison of experimental data with theory in comparison with previously published data.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Sackmann E. Supported membranes: scientific and practical applications. Science. 1996;271:43–48. - PubMed
-
- Alessandrini A., Facci P. Unraveling lipid/protein interaction in model lipid bilayers by atomic force microscopy. J. Mol. Recognit. 2011;24:387–396. - PubMed
-
- Alessandrini A., Seeger H.M., Facci P. What do we really measure in AFM punch-through experiments on supported lipid bilayers? Soft Matter. 2011;7:7054–7064.
-
- Alessandrini A., Facci P. AFM: a versatile tool in biophysics. Meas. Sci. Technol. 2005;16:R65–R92.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

