Phage display-directed discovery of LEDGF/p75 binding cyclic peptide inhibitors of HIV replication
- PMID: 22828501
- PMCID: PMC3498793
- DOI: 10.1038/mt.2012.132
Phage display-directed discovery of LEDGF/p75 binding cyclic peptide inhibitors of HIV replication
Erratum in
-
Phage Display-Directed Discovery of LEDGF/p75 Binding Cyclic Peptide Inhibitors of HIV Replication.Mol Ther. 2021 Feb 3;29(2):887. doi: 10.1016/j.ymthe.2020.12.021. Epub 2021 Jan 1. Mol Ther. 2021. PMID: 33385332 Free PMC article. No abstract available.
Abstract
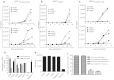
The interaction between the human immunodeficiency virus (HIV) integrase (IN) and its cellular cofactor lens epithelium-derived growth factor (LEDGF/p75) is crucial for HIV replication. While recently discovered LEDGINs inhibit HIV-1 replication by occupying the LEDGF/p75 pocket in IN, it remained to be demonstrated whether LEDGF/p75 by itself can be targeted. By phage display we identified cyclic peptides (CPs) as the first LEDGF/p75 ligands that inhibit the LEDGF/p75-IN interaction. The CPs inhibit HIV replication in different cell lines without overt toxicity. In accord with the role of LEDGF/p75 in HIV integration and its inhibition by LEDGINs, CP64, and CP65 block HIV replication primarily by inhibiting the integration step. The CPs retained activity against HIV strains resistant to raltegravir or LEDGINs. Saturation transfer difference (STD) NMR showed residues in CP64 that strongly interact with LEDGF/p75 but not with HIV IN. Mutational analysis identified tryptophan as an important residue responsible for the activity of the peptides. Serial passaging of virus in the presence of CPs did not yield resistant strains. Our work provides proof-of-concept for direct targeting of LEDGF/p75 as novel therapeutic strategy and the CPs thereby serve as scaffold for future development of new HIV therapeutics.
Figures




References
-
- Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M.et al. (2005Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity Antimicrob Agents Chemother 494721–4732. - PMC - PubMed
-
- Sayana S., and, Khanlou H. Maraviroc: a new CCR5 antagonist. Expert Rev Anti Infect Ther. 2009;7:9–19. - PubMed
-
- Busschots K, De Rijck J, Christ F., and, Debyser Z. In search of small molecules blocking interactions between HIV proteins and intracellular cofactors. Mol Biosyst. 2009;5:21–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

