Reward sensitization: effects of repeated nicotine exposure and withdrawal in mice
- PMID: 22828747
- PMCID: PMC3473332
- DOI: 10.1038/npp.2012.130
Reward sensitization: effects of repeated nicotine exposure and withdrawal in mice
Abstract
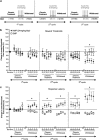
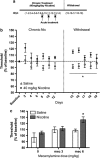
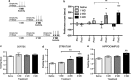
Tobacco dependence is an addiction with high rates of relapse, resulting in multiple quit attempts in individuals who are trying to stop smoking. How these multiple cycles of smoking and withdrawal contribute to nicotine dependence, long-term alterations in brain reward systems, and nicotine receptor regulation is unknown. Therefore, to evaluate how multiple exposures of nicotine and withdrawal periods modulate rewarding properties of nicotine, we used intracranial self-stimulation to measure alterations in the threshold of brain stimulation reward. In addition, we employed the conditioned place preference (CPP) paradigm to evaluate positive context conditioning following each withdrawal period and measured levels of neuronal nicotinic receptors in cortex, striatum, and hippocampus. We found that repeated nicotine exposure and withdrawal enhanced brain stimulation reward and reward sensitivity to acute injections of nicotine. This increased reward was reflected by enhanced CPP to nicotine. Chronic nicotine is known to up-regulate nAChRs (nicotinic acetylcholine receptors) and we found that this up-regulation was maintained for up to 8 days of withdrawal in the striatum and in the hippocampus, but not in the cortex, of animals exposed to multiple nicotine exposure and withdrawal periods. These results demonstrate that repeated exposures to nicotine, followed by withdrawal, induce a persistent increase in both brain reward function and sensitivity to the hedonic value of nicotine and long-lasting up-regulation of neuronal nicotinic receptors. Together, these data suggest that a continuing increase in brain reward function and enhanced sensitivity to nicotine reward following repeated withdrawal periods may be one reason why smokers relapse frequently.
Figures

References
-
- Aggarwal M, Wickens JR. A role for phasic dopamine neuron firing in habit learning. Neuron. 2011;72:892–894. - PubMed
-
- Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. - PubMed
-
- Changeux JP. Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat Rev Neurosci. 2010;11:389–401. - PubMed
-
- Collins AC, Luo Y, Selvaag S, Marks MJ. Sensitivity to nicotine and brain nicotinic receptors are altered by chronic nicotine and mecamylamine infusion. J Pharmacol Exp Ther. 1994;271:125–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

