AV-65, a novel Wnt/β-catenin signal inhibitor, successfully suppresses progression of multiple myeloma in a mouse model
- PMID: 22829079
- PMCID: PMC3256754
- DOI: 10.1038/bcj.2011.41
AV-65, a novel Wnt/β-catenin signal inhibitor, successfully suppresses progression of multiple myeloma in a mouse model
Abstract
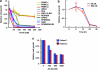
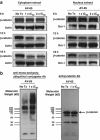
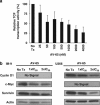
Multiple myeloma (MM) is a malignant neoplasm of plasma cells. Although new molecular targeting agents against MM have been developed based on the better understanding of the underlying pathogenesis, MM still remains an incurable disease. We previously demonstrated that β-catenin, a downstream effector in the Wnt pathway, is a potential target in MM using RNA interference in an in vivo experimental mouse model. In this study, we have screened a library of more than 100 000 small-molecule chemical compounds for novel Wnt/β-catenin signaling inhibitors using a high-throughput transcriptional screening technology. We identified AV-65, which diminished β-catenin protein levels and T-cell factor transcriptional activity. AV-65 then decreased c-myc, cyclin D1 and survivin expression, resulting in the inhibition of MM cell proliferation through the apoptotic pathway. AV-65 treatment prolonged the survival of MM-bearing mice. These findings indicate that this compound represents a novel and attractive therapeutic agent against MM. This study also illustrates the potential of high-throughput transcriptional screening to identify candidates for anticancer drug discovery.
Figures



References
-
- Kuehl WM, Bergsagel PL. Multiple myeloma: evolving genetic events and host interactions. Nat Rev Cancer. 2002;2:175–187. - PubMed
-
- Harousseau JL, Moreau P. Autologous hematopoietic stem-cell transplantation for multiple myeloma. N Engl J Med. 2009;360:2645–2654. - PubMed
-
- Moreau P, Hullin C, Garban F, Yakoub-Agha I, Benboubker L, Attal M, et al. Tandem autologous stem cell transplantation in high-risk de novo multiple myeloma: final results of the prospective and randomized IFM 99-04 protocol. Blood. 2006;107:397–403. - PubMed
-
- Rosinol L, Perez-Simon JA, Sureda A, de la Rubia J, de Arriba F, Lahuerta JJ, et al. A prospective PETHEMA study of tandem autologous transplantation versus autograft followed by reduced-intensity conditioning allogeneic transplantation in newly diagnosed multiple myeloma. Blood. 2008;112:3591–3593. - PubMed
-
- Bringhen S, Avonto I, Magarotto V, Boccadoro M, Palumbo A. Investigational treatments for multiple myeloma. Expert Opin Investig Drugs. 2006;15:1565–1582. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

