Donepezil for dementia with Lewy bodies: a randomized, placebo-controlled trial
- PMID: 22829268
- PMCID: PMC3504981
- DOI: 10.1002/ana.23557
Donepezil for dementia with Lewy bodies: a randomized, placebo-controlled trial
Abstract
Objective: Because cholinergic deficits are prominent in dementia with Lewy bodies (DLB), we investigated the effects of a cholinesterase inhibitor, donepezil, in such patients in a randomized, double-blind, placebo-controlled exploratory phase 2 trial.
Methods: One-hundred forty patients with DLB, recruited from 48 specialty centers in Japan, were randomly assigned to receive placebo or 3, 5, or 10 mg of donepezil hydrochloride daily for 12 weeks (n = 35, 35, 33, and 37, respectively). Effects on cognitive function were assessed using the Mini-Mental State Examination (MMSE) and several domain-specific neuropsychological tests. Changes in behavior were evaluated using the Neuropsychiatric Inventory, caregiver burden using the Zarit Caregiver Burden Interview, and global function using the Clinician's Interview-Based Impression of Change-plus Caregiver Input (CIBIC-plus). Safety measures included the Unified Parkinson's Disease Rating Scale part III.
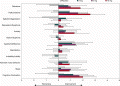
Results: Donepezil at 5 and 10 mg/day was significantly superior to placebo on both the MMSE (5 mg: mean difference, 3.8; 95% confidence interval [CI], 2.3-5.3; p < 0.001; 10 mg: mean difference, 2.4; 95% CI, 0.9-3.9; p = 0.001) and CIBIC-plus (p < 0.001 for each); 3 mg/day was significantly superior to placebo on CIBIC-plus (p < 0.001), but not on the MMSE (p = 0.017). Significant improvements were found also in behavioral measures (p < 0.001) at 5 and 10 mg/day and caregiver burden (p = 0.004) at 10 mg/day. The safety results were consistent with the known profile of donepezil and similar among groups.
Interpretation: Donepezil at 5 and 10mg/day produces significant cognitive, behavioral, and global improvements that last at least 12 weeks in DLB patients, reducing caregiver burden at the highest dose. Donepezil is safe and well tolerated.
Copyright © 2012 American Neurological Association.
Figures

References
-
- McKeith I, Mintzer J, Aarsland D. Dementia with Lewy bodies. Lancet Neurol. 2004;3:19–28. - PubMed
-
- McKeith IG, Galasko D, Kosaka K. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. Neurology. 1996;47:1113–1124. - PubMed
-
- McKeith IG, Rowan E, Askew K. More severe functional impairment in dementia with Lewy bodies than Alzheimer disease is related to extrapyramidal motor dysfunction. Am J Geriatr Psychiatry. 2006;14:582–588. - PubMed
-
- Allan L, McKeith I, Ballard C, Kenny RA. The prevalence of autonomic symptoms in dementia and their association with physical activity, activities of daily living and quality of life. Dement Geriatr Cogn Disord. 2006;22:230–237. - PubMed
-
- McKeith IG, Dickson DW, Lowe J. Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology. 2005;65:1863–1872. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

